Cladosporium
Cladosporium species are ubiquitous worldwide, and commonly isolated from soil and organic matter. They represent the most frequently isolated airborne fungi.
The genus has undergone a number of revisions. The well-known thermotolerant ‘true human-pathogenic species, formerly known as C. bantiana, C. carrionii and C. devriesii, characterised by the absence of conidiophores, and unpigmented conidial scars, were reclassified in Cladophialophora (de Hoog et al. 1995, Bensch et al. 2012). The remaining species of medical interest were C. cladosporioides, C. herbarum, C. oxysporum, and C. sphaerospermum. More recently, extensive revisions based on polyphasic approaches have recognised 169 species, and demonstrated that C. cladosporioides, C. herbarum and C. sphaerospermum are species complexes encompassing several sibling species that can only be distinguished by phylogenetic analyses (Crous et al. 2007, Schubert et al. 2007, Zalar et al. 2007, Bensch et al. 2010, 2012).

Culture of Cladosporium cladosporioides.
Sandoval-Denis et al. (2015) analysed 92 clinical isolates from the United States using phenotypic and molecular methods, which included sequence analysis of the ITS and D1/D2 regions, partial EF-1α and actin genes. Surprisingly, the most frequent species was Cladosporium halotolerans (15%), followed by C. tenuissimum (10%), C. subuliforme (6%), and C. pseudocladosporioides (5%). However, 40% of the isolates did not correspond to any known species and were deemed to represent at least 17 new lineages for Cladosporium. The most frequent anatomic site of isolation was the respiratory tract (55%), followed by superficial (28%) and deep tissues and fluids (15%). Species of the two recently described Cladosporium-like genera Toxicocladosporium and Penidiella were also reported for the first time from clinical samples (Sandoval-Denis et al. 2015).
RG-1 organisms.
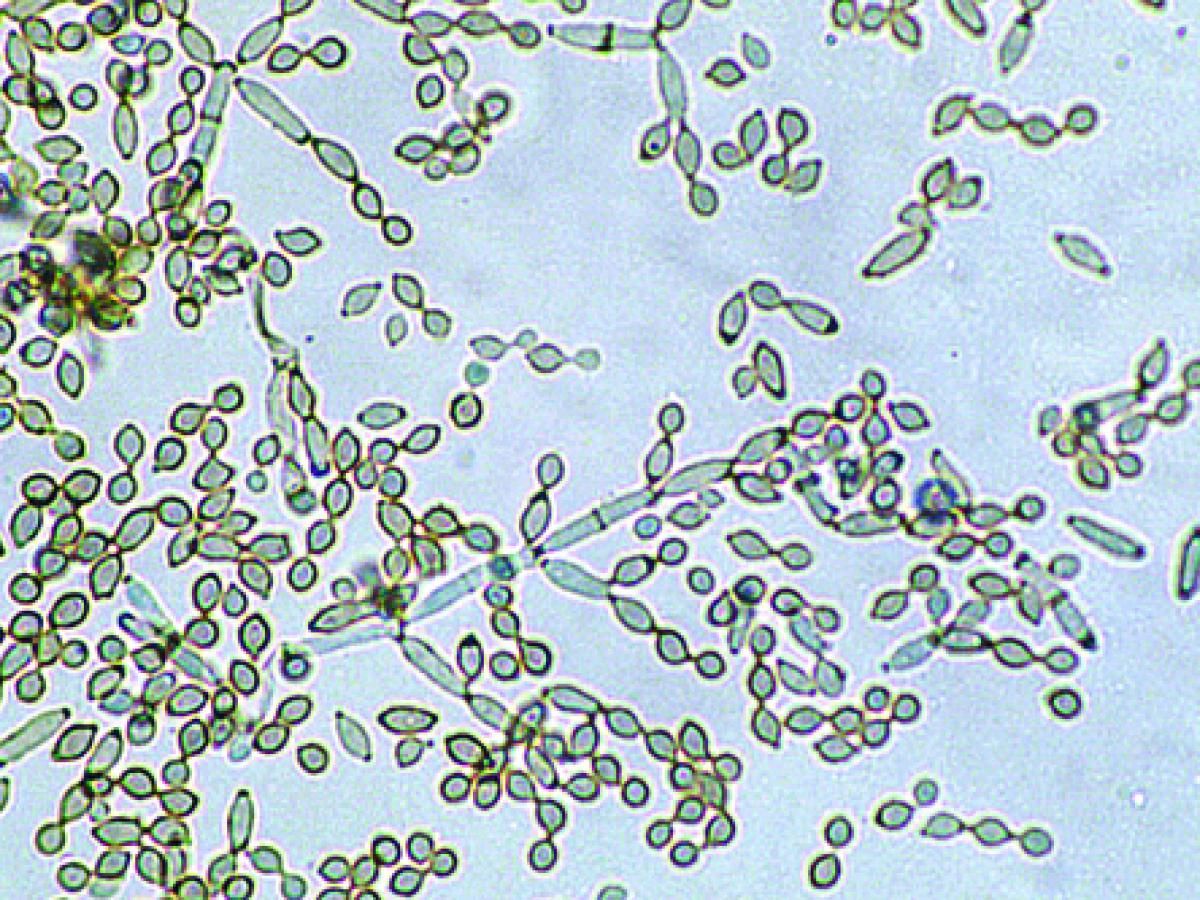
Conidiophores and conidia of Cladosporium cladosporioides.
Morphological description:
Colonies are slow growing, mostly olivaceous-brown to blackish-brown but also sometimes grey, buff or brown, suede-like to floccose, often becoming powdery due to the production of abundant conidia. The reverse is olivaceous-black. Vegetative hyphae, conidiophores and conidia are equally pigmented. Conidiophores are more or less distinct from the vegetative hyphae, being erect, straight or flexuose, unbranched or branched only in the apical region, with geniculate sympodial elongation in some species. Conidia are produced in branched acropetal chains, being smooth, verrucose or echinulate, one to four-celled, and have a distinct dark hilum. The term blastocatenate is often used to describe chains of conidia where the youngest conidium is at the apical or distal end of the chain. Note: The conidia closest to the conidiophore, and where the chains branch, are usually “shield-shaped”. The presence of shield-shaped conidia, a distinct hilum, and chains of conidia that readily disarticulate, are characteristic of the genus Cladosporium.
Key features:
Dematiaceous hyphomycete forming branched acropetal chains of conidia, each with a distinct hilum.
Molecular identification:
Genus level identification is usually sufficient and morphological identification can be confirmed by ITS and D1/D2 sequence analysis. Multilocus gene analysis of the ITS, D1/D2, EF-1α and actin gene loci is necessary for accurate species identification (Bensche et al. 2012).
| No | <0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ≥16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AmB | 27 | 3 | 3 | 12 | 9 | ||||||
| ISAV | 21 | 1 | 2 | 3 | 5 | 2 | 3 | 5 | |||
| VORI | 27 | 1 | 4 | 5 | 5 | 4 | 4 | 4 | |||
| POSA | 27 | 1 | 1 | 5 | 12 | 5 | 2 | 1 | |||
| ITRA | 27 | 1 | 4 | 7 | 13 | 1 | 1 |
References:
Ellis (1971, 1976), Domsch et al. (1980), McGinnis (1980), de Hoog et al. (2000, 2015), Crous et al. (2007), Schubert et al. (2007), Zalar et al. (2007), Bensch et al. (2010 and 2012), Sandoval-Denis et al. (2015).
