Scedosporium
The taxonomy of this genus has been subject to change on the basis of sequence data; Scedosporium apiospermum and Scedosporium boydii (formerly Pseudallescheria boydii) are now recognised as separate species and along with S. aurantiacum are the principal human pathogens (Lackner et al. 2014a).
The majority of infections are mycetomas, the remainder include infections of the eye, ear, central nervous system, internal organs and more commonly the lungs. Scedosporium dehoogii and S. minutispora are mainly isolated from environmental samples and have been rarely reported from clinical cases (Gilgado et al. 2005, Rainer and de Hoog 2006, Cortez et al. 2008, Kaltseis et al. 2009).
Scedosporium prolificans has been transferred to the genus Lomentospora. L. prolificans is phylogenetically and morphologically distinct from the remaining Scedosporium species (Lennon et al. 1994, Lackner et al. 2014a).
Morphological identification of Scedosporium species has become increasingly unreliable and molecular identification methods are now recommended. The conidial states of S. apiospermum and S. boydii are morphologically indistinguishable; although the latter is homothallic and produces ascocarps. S. aurantiacum also exhibits similar conidial morphology but most strains produce a pale to bright yellow diffusible pigment on potato dextrose agar.
Molecular identification:
Recommended genetic markers are ITS and β-tubulin (Lackner et al. 2012a).
MALDI-TOF MS:
A comprehensive ‘in-house’ database of reference spectra allows accurate identification of Scedosporium and Lomentospora species (Lau et al. 2013, Sitterlé et al. 2014).
References:
McGinnis (1980), Domsch et al. (1980), McGinnis et al. (1982), Campbell and Smith (1982), Rippon (1988), de Hoog et al. (2000, 2015), Gilgado et al. (2005), Rainer and de Hoog (2006), Guarro et al. (2006), Lackner et al. (2014a).
Species descriptions
-
Scedosporium apiospermum
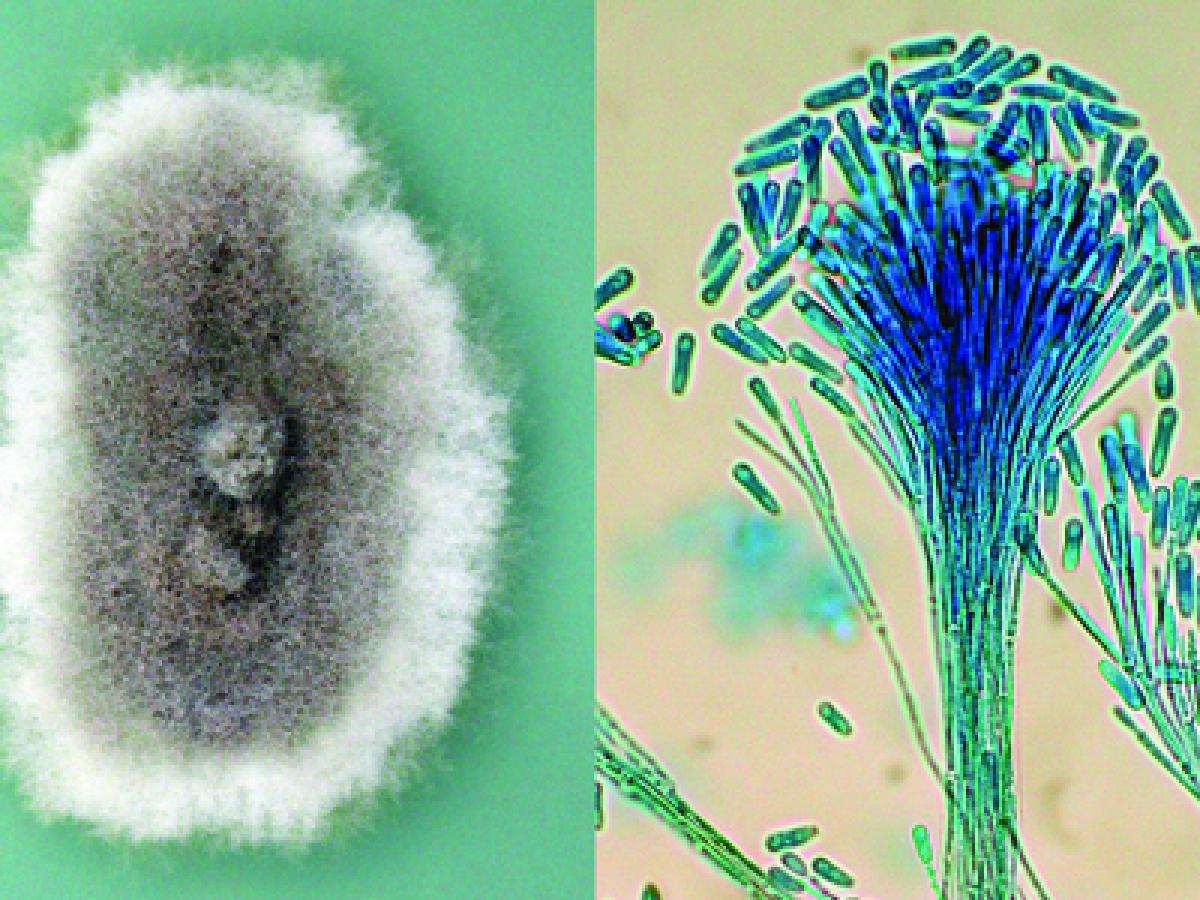
Culture and synnemata of Scedosporium apiospermum.
Synonymy:
Pseudallescheria apiospermaRG-2 organism.
Morphological description:
Colonies are fast growing, greyish-white, suede-like to downy with a greyish-black reverse. Numerous single-celled, pale-brown, broadly clavate to ovoid conidia, 4-9 x 6-10 µm, rounded above with truncate bases are observed. Conidia are borne singly or in small groups on elongate, simple or branched conidiophores or laterally on hyphae.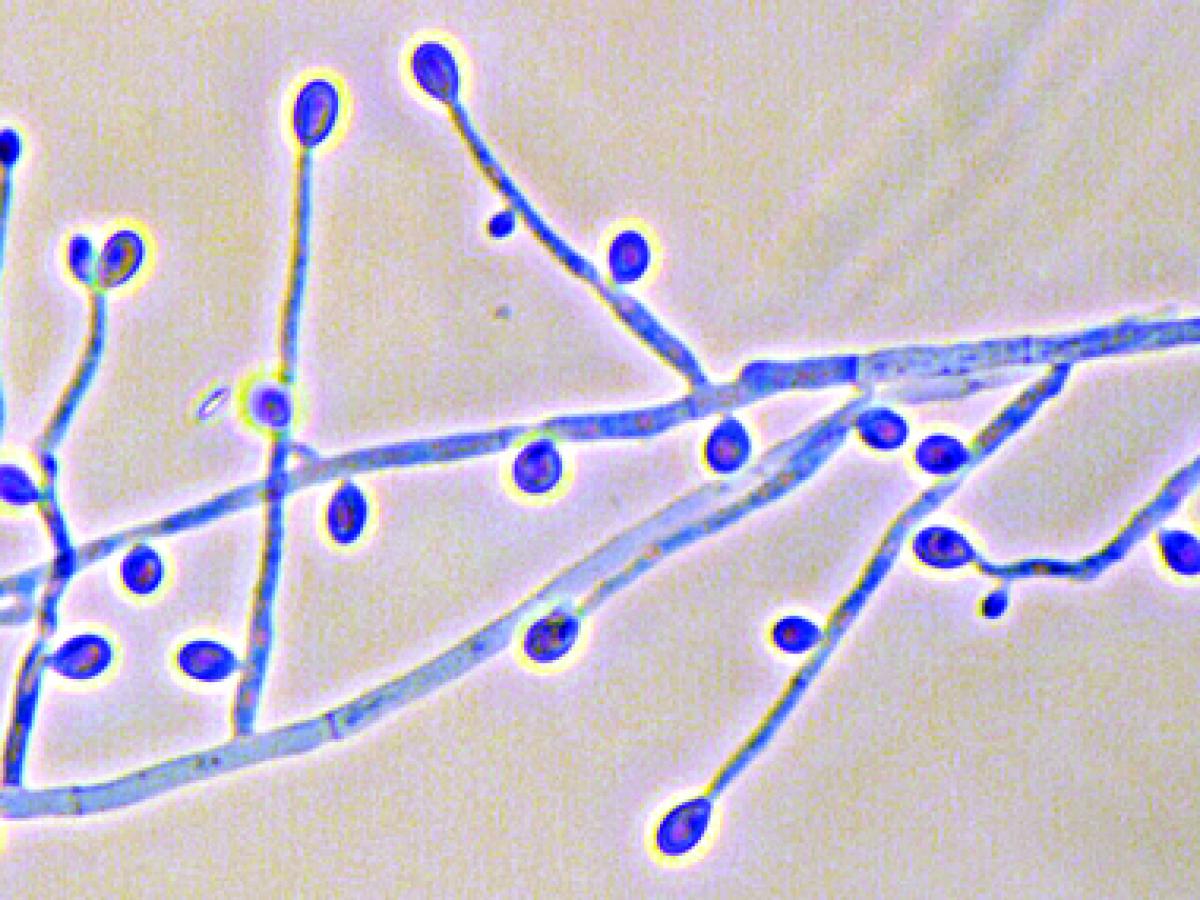
Scedosporium apiospermum conidiophores and conidia.
Conidial development can be described as annellidic, although the annellations (ring-like scars left at the apex of an annellide after conidial secession) are extremely difficult to see. Erect synnemata may be present in some isolates. Optimum temperature for growth is 30-37C.
Antifungal susceptibility: Scedosporium apiospermum (Australian national data); MIC µg/mL. No ≤0.03 0.06 0.125 0.25 0.5 1 2 4 8 16 ≥32 AmB 484 1 4 14 69 122 257 17 ISAV 135 1 9 23 37 45 20 VORI 480 6 31 93 146 173 27 1 1 2 POSA 429 3 6 41 112 205 59 1 2 ITRA 484 2 9 56 141 211 36 10 2 17 -
Scedosporium auranticum
Culture reverse (PDA) of S. apiospermum (left) and S. aurantiacum (right) showing production of a light yellow diffusible pigment that is typical of S. aurantiacum. Conidiophores and conidia of S. aurantiacum.
RG-2 organism.
Morphological description:
Most isolates produce a light yellow diffusible pigment on potato dextrose agar after a few days incubation. Conidiogenous cells and conidia are similar in shape and size to S. apiospermum, and the two can best be distinguished by genetic analysis. Conidiogenous cells arising from undifferentiated hyphae are cylindrical to slightly flask-shaped, producing slimy heads of one-celled , smooth-walled, subhyaline, obovoid or sub-cylindrical conidia. 5-14 x 2-5 um. Erect synnemata may be present in some isolates, but the teleomorph is unknown. Optimum temperature for growth 37-40C, max 45CAntifungal susceptibility: Scedosporium aurantiacum (Australian national data); MIC µg/mL. No ≤0.03 0.06 0.125 0.25 0.5 1 2 4 8 ≥16 AmB 48 1 1 7 39 ISAV 27 1 1 3 14 8 VORI 48 7 11 22 6 1 1 POSA 48 5 30 10 3 ITRA 48 5 25 10 1 7 -
Scedosporium boydii
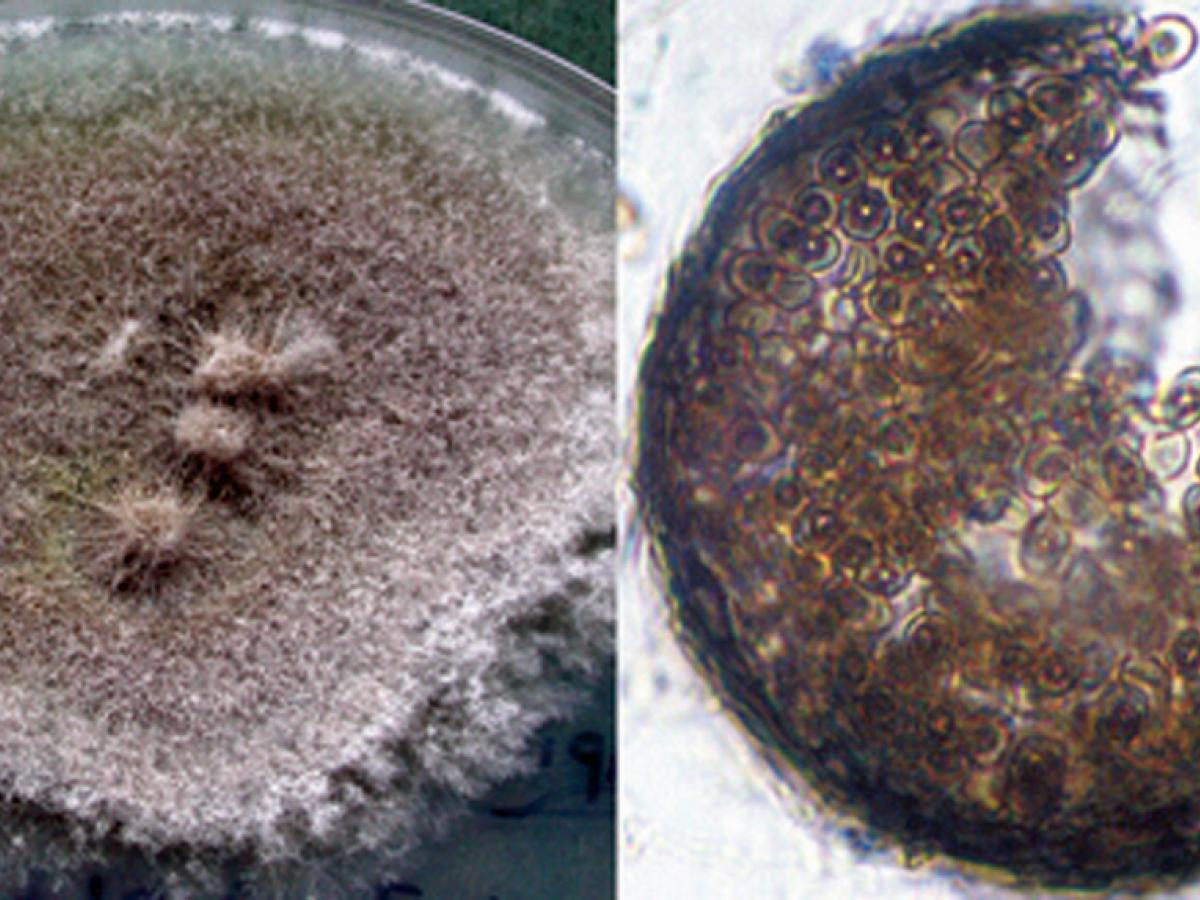
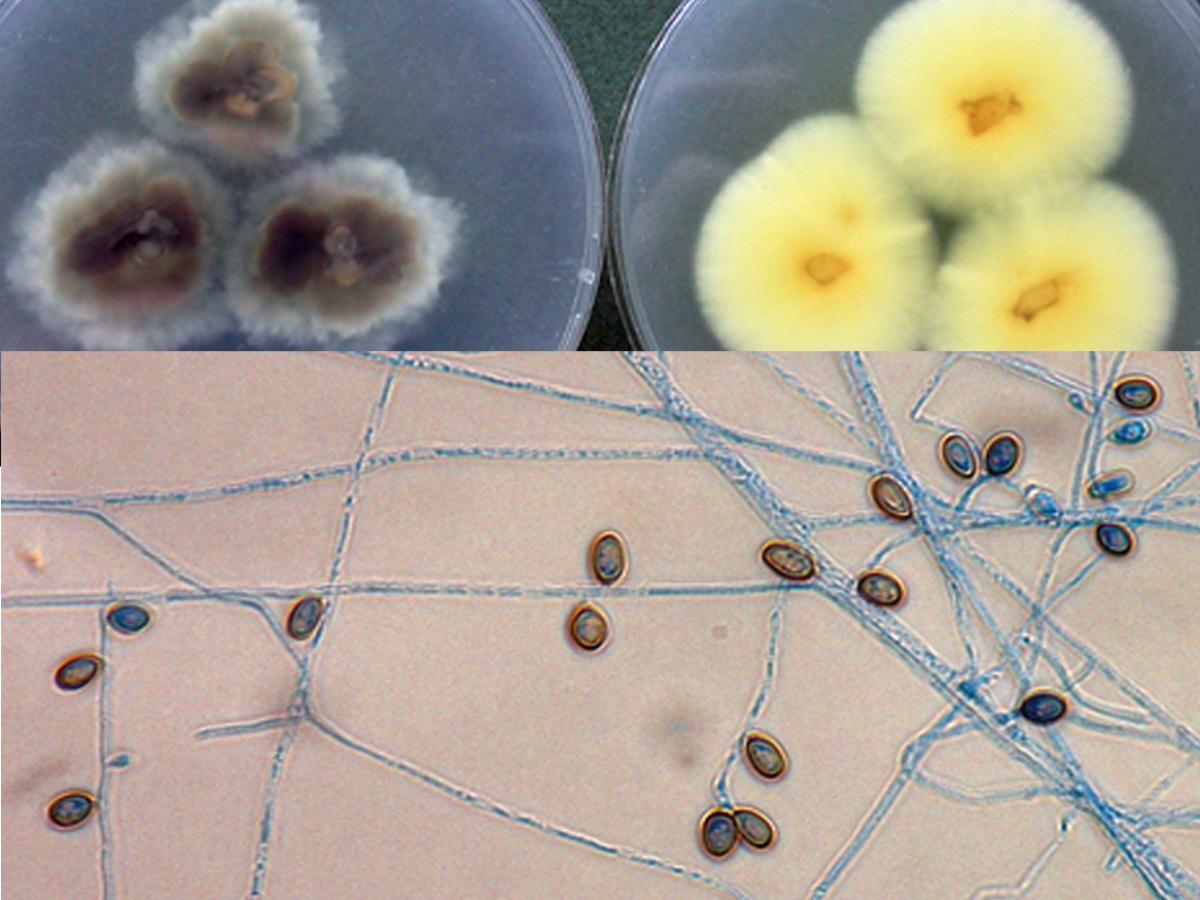
Culture and cleistothecium of Scedosporium boydii.
Synonymy:
Pseudallescheria boydii.RG-2 organism.
Morphological description:
Colonies are fast growing, greyish-white, suede-like to downy with a greyish-black reverse. Numerous single-celled, pale-brown, broadly clavate to ovoid conidia, 4-9 x 6-10 µm, rounded above with truncate bases are observed. Conidia are borne singly or in small groups on elongate, simple or branched conidiophores or laterally on hyphae. Cleistothecia (non-ostiolate ascocarps) are yellow-brown to black, spherical, 50-200 μm in diameter, and are mostly submerged in the agar and are composed of irregularly interwoven brown hyphae. When crushed cleistothecia release numerous, faintly brown, ellipsoidal ascospores, 4-5 x 7- 9 µm in size. Erect synnemata may be present in some isolates. Optimum temperature for growth is 30-37C.Note: S. boydii is homothallic and is recognised by smaller cleistothecia (50-200 μm) whereas S. apiospermum is heterothallic (requires mating of two strains) and has larger cleistothecia, 140-480 μm (Gilgado et al. 2010).
Antifungal susceptibility: Scedosporium boydii (Australian national data); MIC µg/mL. No ≤0.03 0.06 0.125 0.25 0.5 1 2 4 8 ≥16 AmB 11 1 10 ISAV 5 4 1 VORI 11 1 2 7 1 POSA 11 2 7 2 ITRA 11 2 8 1 Antifungal susceptibility: Scedosporium boydii vs. S. apiospermum (Lackner et al., 2014b); MIC µg/mL.
Antifungal
Scedosporium boydii
Scedosporium apiospermum
Range
MIC90
Range
MIC90
AmB
0.5->16
≥16
0.5->16
≥16
VORI
0.125-2
2
0.25->8
2
POSA
0.125->16
≥16
0.25->16
≥16
ITRA
0.125->16
≥16
0.25->16
≥16
