Personal & Societal Health
-
Breath analysis for real-time diagnosis

A hand-held breath analysis device has the potential to revolutionise global health and improve accessibility to quality health services. By detecting diseases early through breath biomarkers and monitoring known diseases, it can offer quick, non-invasive screening for personalised treatment anywhere at any time.
Dr Sarah Scholten, an IPAS Postdoctoral Researcher with the Precision Measurement Group, leads the Breath Analysis Research. Every breath we take holds the potential to reveal profound insights into our well-being. Dr Scholten is looking into a revolutionary approach using optical frequency comb technology, a Nobel Prize-winning innovation.
Laser spectroscopy, the technique employed, delves into the composition of exhaled breath, detecting biomarker molecules associated with specific health conditions in real time. For example, elevated acetone levels may indicate diabetes, while increased levels of ammonia can be associated with liver disorders.
The optical frequency comb, known as the world's most precise ruler, enables instant analysis of breath samples' spectral signatures. Advanced computational methods can detect molecular concentrations at extremely low levels, instantly revealing the unique molecular makeup of a breath.
However, optical frequency combs are currently found in specialised labs because they are big, complex, and expensive. The ARC Centre of Excellence in Optical Microcombs for Breakthrough Science is focused on making this technology smaller and cheaper. More importantly, making it accessible to all. Sarah's breath analysis method and innovation, combined with COMBS's promised accessibility, could be made into a portable device allowing for wellness assessment in real-time in remote areas with vulnerable populations that do not have access to state-of-the-art facilities.
See the Molecular Spectroscopy Website -
Ultrathin endoscope for hearth disease diagnosis

Throughout a lifetime, plaques accumulate in our arteries. While most stay stable, some can rupture and block arteries, leading to serious health risks. Current diagnostic tools lack the accuracy to reliably predict which plaques might cause issues, often resulting in costly overtreatment or sudden death.
Dr Jiawen Li, an Associate Professor at the University of Adelaide and renowned innovator in biomedical engineering has engineered a groundbreaking device to help cardiologists identify patients at the greatest risk of heart attack. Her ultrathin 3D-printed endoscope is designed to probe inside blood vessels and generate high-quality images of plaques, providing detailed imaging while fitting safely inside the arteries feeding the heart.
Dr Li’s endoscope utilises advanced light-based imaging techniques, combining optical coherence tomography (OCT) and 3D nano/micro-manufacturing. OCT is a non-invasive technique that uses light waves to capture high-resolution, cross-sectional images of biological tissues, similar to ultrasound but with light. It creates detailed images revealing the internal structure of plaques.
3D nano/micro-manufacturing is used for creating extremely small and precise structures. Dr Li and her collaborators in Stuttgart developed a way to print a lens directly onto an optical fibre as thin as a human hair, allowing the lens to navigate through arteries to the heart and provide real-time, high-resolution images from within blood vessels.
By integrating OCT with 3D nano/micro-manufacturing, Dr Li’s endoscope offers a powerful diagnostic tool. It can safely navigate through the narrow pathways of arteries, capturing detailed images and molecular information about plaque composition. This minimally invasive approach reaches critical areas around the heart without damaging artery walls, enabling clinicians to identify plaques most likely to rupture and cause medical issues.
Dr Li’s endoscope has the potential to revolutionise heart disease diagnosis and treatment by providing high-resolution images and molecular data. Precisely identifying high-risk plaques can lead to more targeted and effective treatments, reducing overtreatment and the risk of sudden heart attacks. Additionally, this technology holds promise for detecting other diseases, such as cancer, in hard-to-image areas like the bile duct and lungs.
-
Novel fluorescence research for asbestos detection

Asbestos has been banned in Australia for more than 20 years, but is a continuing threat to health and wellbeing due to legacy use, presence in mining ores, and accidental imports. Asbestos is difficult to discern from other fibrous materials in the field, and currently identifying asbestos at building sites requires sending a sample to a specialised laboratory, costing time and money. This cost weighs into risk/benefit decision-making when an unidentified sample is found, particularly for home renovators who are not practiced at identifying asbestos-containing materials. Peak asbestos use in homes in Australia was in the 1960s and 1970s; as these houses age and require repair and replacement the risk grows of a second wave of asbestos-related disease.
The Prescott Environmental Luminescence Laboratories is looking to solve this problem. The Asbestos Novel Fluorescence Team, led by Dr Erik Shartner, under Professor Nigel Spooner’s supervision, have partnered with the Australian Institute for Machine Learning (AIML) to produce a fluorescence-based asbestos sensor. This device will be portable, affordable, and be able to produce instantaneous results in the field.
Many building materials fluoresce (produce a different colour of light when exposed to light), including asbestos; however, each material reacts uniquely under different excitation conditions. The PELL group is providing a large training data set of asbestos, non-asbestos, and mixed media samples fluorescing under different conditions to train an asbestos-sensing machine learning model.
“Does this contain asbestos” is a question that will save lives if answered more quickly and more cheaply. Luckily, PELL and AIML are on the case!
More information on the PELL Group website and AIML's website.
-
Optical imaging technology for enhanced IVF success

A passion for helping hopeful parents facing fertility issues has driven Associate Professor Kylie Dunning in her pioneering research into IVF. Motivated by creating a world where fewer couples struggle with infertility, an often invisible and stigmatised health challenge faced by more than 15% of Australians.
With personal experience of the challenges and heartache of starting a family, Associate Professor Dunning is paving the way for patients to experience better and more effective fertility care, through the creation of exciting new technologies that will spearhead a revolution in IVF practices.
One of the greatest challenges for IVF clinics is identifying which embryos are suitable for transfer back into the patient. Overcoming this challenge would increase the number of patients taking home a baby. The current gold standard technology involves taking a small number of cells from the embryo (known as biopsy), an invasive procedure, and then sequencing the DNA to confirm that the embryo has the predicted number of chromosomes, a process known as pre-implantation genetic testing. As well as being invasive, this procedure is inaccurate in many cases.
"We know that aneuploidy, or the presence of cells with a divergent number of chromosomes, is quite common in human embryos. We also know they’re often mosaic, meaning the embryo has some normal cells, and some aneuploid cells. This reduces the chances of a successful pregnancy"Associate Professor Kylie Dunning
New technology being developed by Associate Professor Dunning and her team overcomes the need for a cell biopsy, instead using light to take a non-invasive ‘molecular photo’ to assess the health of the embryo.
Dr Dunning’s revolutionary procedure involves shining very gentle doses of light upon an embryo, and using the scattered light that comes back to reveal the intricacies of its biochemistry.
Currently at the pre-clinical stage, these discoveries will, Dr Dunning hopes, one day help choose the best embryos for transfer, reducing costs and heartache for hopeful parents.Excitingly, the same procedures also extend into the realm of IVF in agriculture. This will lead to improved farming practices ensuring food availability and affordability on a global scale.
Learn more about Associate Professor Kylie Dunning’s work:
LinkedIn | Google Scholar | Media | YouTube -
Advanced imaging for improved diabetic foot ulcer care
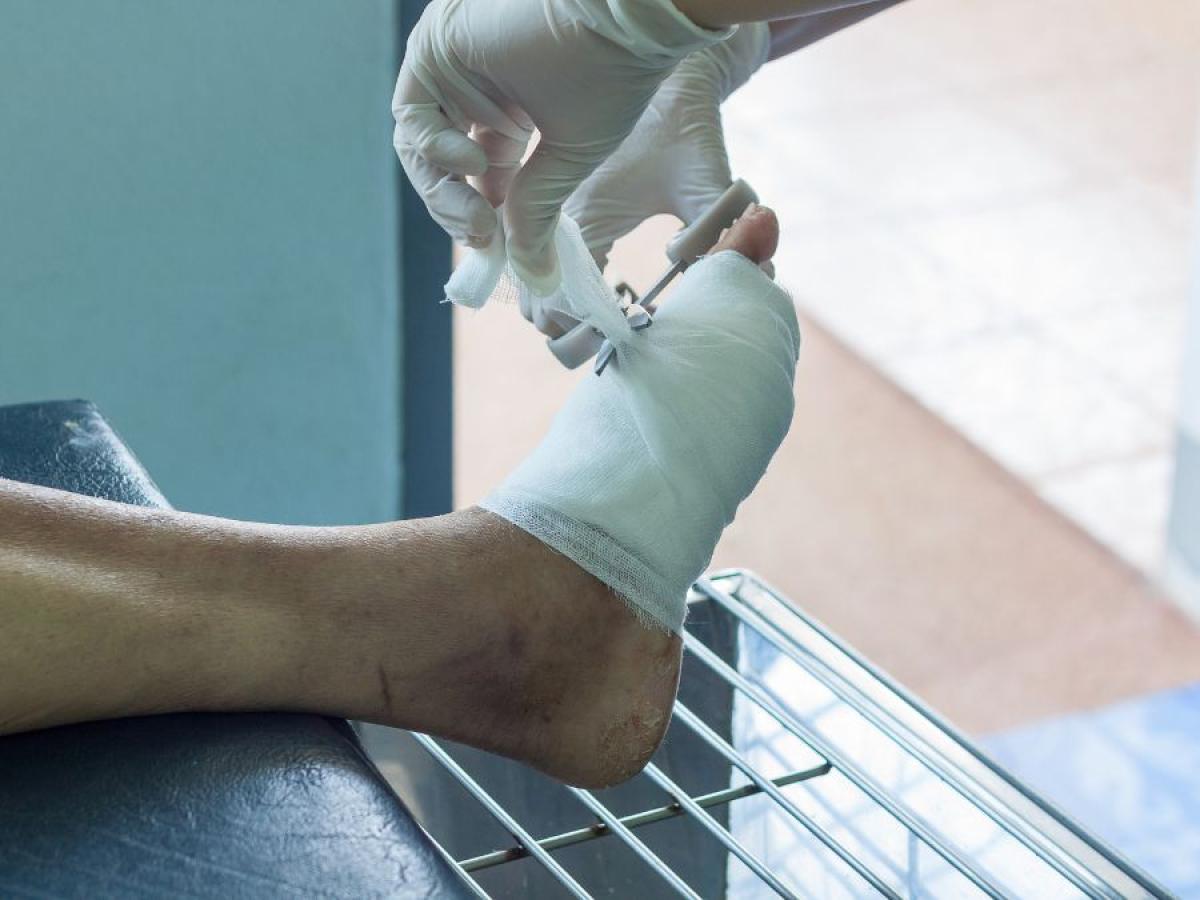
Diabetes affects 1 in 11 adults, and the prevalence is increasing. A common complication of diabetes is diabetic foot ulcers. These are wounds on the foot that often take months to heal. In some cases, they can lead to foot amputations.
Prof Robert McLaughlin and his team are developing new imaging technologies to help doctors choose the best treatments for patients with diabetic foot ulcers.
The team have developed a technique using light to see under the skin. It can measure the tiny blood vessels that supply oxygen and nutrients to the tissue. These micro-blood vessels, as thin as a human hair, are a critical part of our body’s defence system to heal foot ulcers. In diabetes, these blood vessels break down. By measuring the health of these blood vessels, doctors may be able to identify which wounds will heal, and which should be sent off for surgery immediately.
Their new approach uses a technology called optical coherence tomography, which can measure the tiny movements of red blood cells in the vessels. This microscopic-scale data is then analysed using AI algorithms to give doctors a measure of skin's health. The team will undertake clinical trials of this new technology over 2024 – 2025.
The project is supported by a Commonwealth Department of Education translational research grant through Australia’s Economic Accelerator program.
See the Bioengineering Imaging Group's website -
New targeted treatment for chronic nerve pain
Chronic pain affects more than 3 million Australians, restricting their ability to work and live as well as negatively impacting their mental health.
The overall economic burden of chronic pain in 2020 was estimated to be $144.1 billion and is projected to reach up to $215.6 billion in 2050. IPAS Research Leader Prof Andrew Abell and his team have developed a cutting-edge pharmaceutical treatment for a type of chronic pain called neuropathic pain.
Neuropathic pain, also known as nerve pain, is caused by disease, damage, or dysfunction of the nervous system. Current treatments are greatly limited by poor efficacy, side-effect profiles, addiction and withdrawal, demonstrating that improved neuropathic pain drugs are desperately required.
Prof Abell and his team harnessed the high levels of reactive oxygen species (ROS) at sites of neuropathic pain by incorporating a molecular ROS sensor into a drug that treats the underlying nerve damage and alleviates pain. The resulting drug is a targeted treatment that is only active when and where it is required. This ultimately creates a safer and better-tolerated drug that is non-addictive, non-sedative and without susceptibility to withdrawal or drug tolerance.
This work is a collaboration between the Abell group and A/Prof Peter Grace at The University of Texas MD Anderson Cancer Center and has attracted 2.4 million USD in funding from the National Institute of Health’s HEAL Initiative. Efforts to commercialise this research are also being undertaken by Immunologic Inc., a company formed in the US involving Abell group members.
-
Glass to help improve medical instruments accuracy

Researchers Mingze Yang, Dr Yunle Wei, Dr Philipp Reineck, Professor Heike Ebendorff-Heidepriem, Associate Professor Jiawen Li, and Professor Robert McLaughlin from the University of Adelaide and RMIT University have created a new type of material that mimics how human tissues interact with light. This discovery could change the future of medical imaging and diagnostics, making it possible to use the glass as a basis for instrument calibration.
Medical imaging tools need materials that behave like human tissues to test and improve their accuracy. Traditional materials often degrade over time, limiting their effectiveness and reliability.
The answer is inorganic glass! They use special glass mixed with nickel ions to absorb light like haemoglobin. By heating the glass, crystals form inside, scattering light similarly to human tissues.
Glass samples are stable and consistent, perfect for accurate testing. This method can imitate different types of human tissues, helping improve medical imaging technologies for a broad range of testing. Glass samples can also be manufactured in various shapes and sizes, even using 3D printing for complex designs, making it easier to develop and test new medical devices.
Building on this breakthrough, the team’s latest research applies the same glass phantom technology to brain surgery. In fluorescence-guided neurosurgery, surgeons use special dyes to make tumours glow, but accurate detection depends on well-calibrated imaging systems. The researchers engineered glass that mimics the optical properties of brain tissue and tumour fluorescence. Using silver nanoparticles to replicate blood absorption, microscopic crystals and bubbles to scatter light like tissue, and samarium ions to produce tumour-like glow. These durable, precise phantoms provide a long-lasting standard for testing surgical imaging tools, improving accuracy and safety in the operating theatre.
-
Laser-medical device to kill antibiotic-resistant bacteria

Antimicrobial resistance is a serious global health issue, especially surgical site infections, the second most common hospital-acquired infection in developed countries.
Dr Katharina Richter, PhD’s team is tackling this problem using Far-UVC light to kill bacteria without the harmful effects of traditional UV light.
They are developing a laser device that emits Far-UVC light (200 to 230 nanometers). Unlike conventional UV light, which can damage mammalian DNA and cause cancer, Far-UVC light kills bacteria without harming human cells, making it safe for sterilising surgical sites and treating skin infections.
The science behind this breakthrough lies in the distinct wavelength of Far-UVC light. At 200 to 230 nanometers, the light is absorbed by the outer layers of bacterial cells, leading to their destruction, while being unable to penetrate the protective layers of human skin and eyes. This selective targeting ensures that harmful bacteria are eliminated without posing a risk to human health.
Beyond the operating room, this technology could replace antibiotics, helping to fight antimicrobial resistance. It has potential uses in emergency rooms, general practices, and treating chronic skin conditions like eczema.
This innovative approach could change infection control and reduce antimicrobial-resistant infections, protecting public health for the future.
The Richter Lab is based at the Basil Hetzel Institute for Translational Health Research (BHI) within the Surgical Sciences Group. This positioning offers the lab a unique advantage. BHI serves as the research arm of The Queen Elizabeth Hospital (QEH) and boasts strong links to the University of Adelaide.
-
Plasma activated water to combat superbugs

Plasma activated water
The looming threat of antibiotic-resistant superbugs poses a significant risk to human health, with projections of 10 million annual deaths by 2050. Dr. Katharina Richter and her team are at the forefront of the battle against superbugs, developing innovative treatments to address this global health crisis.
Their weapon? Cold Plasma Technology. This cutting-edge technology is a groundbreaking antibacterial sanitiser with the potential to revolutionise the health and food industries.
Plasma, the fourth state of matter, is achieved by infusing energy into a gas. The Richter lab, in partnership with Plasmatreat, has engineered a process that enriches water with ionised gas and reactive radicals, rendering it antibacterial.
Utilising advanced microscopy techniques, the team can measure the efficacy of plasma-activated water in combating bacterial biofilms. The innovative plasma water rapidly tackles bacteria, leaving behind pure water as radicals are consumed. This environmentally friendly solution generates no harmful waste, making a significant step toward a safer and more sustainable future.
Plasma Activated Water serves a dual purpose:
1- Enhancing infection control and wound care as a viable alternative to increasingly ineffective antibiotics
2- Transforming the food industry with an eco-friendly sanitiser.
Simple Technology, Big Impact
Unlike traditional disinfectants that leave harmful residues, plasma water breaks down into pure water after use. Plus, with proper equipment, plasma water could be generated, used, filtered and reused on-site, making this powerful technology accessible even in resource-limited regions.
This innovative plasma-activated water solution empowers a healthier world, combating superbugs and promoting food safety, all while safeguarding the environment.
Learn more about Dr Katharina Richter's work -
Smart needle for safer and more effective brain surgery

Over 1,600 Australians are diagnosed with brain tumours each year, being the leading cause of cancer-related death in children and in adults over the age of 65. Brain tumours have a low survival rate, with less than half of the patients surviving one year of diagnosis, and only 22% of patients surviving 5 years.
A common part of diagnosis is to perform a needle biopsy, which involves inserting a large needle into the patient’s brain to remove a sample of the tumour. If the needle damages a blood vessel, this can cause a stroke for the patient. 2%-3% of patients undergoing brain biopsy will suffer long-term disability, and 1% will die.
IPAS member and ARC Centre of Excellence for Nanoscale BioPhotonics Senior Investigator Prof Robert McLaughlin and his team have developed a revolutionary new medical device that will make neurosurgery safer.
A tiny imaging probe, encased within a brain biopsy needle, lets surgeons ‘see’ at-risk blood vessels as they insert the needle, allowing them to avoid causing bleeds that can potentially be fatal.
The device contains a tiny fibre-optic camera, the size of a human hair, shining infrared light and allowing the needle to see where it is going. This is combined with smart image processing software to detect vessels and alert the surgeon before the vessels are damaged.
The smart needle has been developed in collaboration with future end-users, clinicians from Sir Charles Gairdner Hospital. In 2016, Professor Christopher Lind, Consultant Neurosurgeon, successfully demonstrated the smart needle in a pilot trial with 12 patients undergoing neurosurgery.
This is the first demonstration in human neurosurgery of a smart needle that enables safer brain surgery. Beyond brain biopsies, this technology has direct applications in other forms of neurosurgery, such as deep brain stimulation where electrode needles are inserted into a patient’s brain to alleviate the symptoms of diseases such as Parkinson’s disease.
The team is now exploring other applications for their ‘smart needle’ technology, both in the biotech and industrial space while working on the next generation of multi-function imaging probes.
As highlighted in the Australian Research Council recent publication Making a difference, Understanding our world and translating fundamental research, “The ‘smart needle’ is an outstanding example of how ARC-funded research can translate into real world benefits—in this case, commercially for the medical technology industry and, ultimately, improved health services for Australians”.
-
Novel imaging technique to detect biomarkers of early-stage ovarian cancer

MALDI-MSI of N-glycans is a relatively new technique that has immense potential in several clinical applications including identification and validation of biomarkers in cancer tissues.
IPAS PhD student Matthew Briggs recently published about using this novel technique to spacially map sugars on ovarian cancer tissue samples.
