Adelaide Research Assay Facility (ARAF)
Adelaide Research Assay Facility provides specialised, high-throughput and high-sensitivity assays of physiologically important analytes for academic researchers and commercial customers Australia-wide.
We provide a cost effective one-stop-shop for researchers who require these analyses but may not have ready access to the expertise, reagents or equipment to undertake them.
We provide services and consultation for academic and commercial clients who require specialised measurements of analytes in human or animal biological fluids or cell culture / tissue extracts. The services we offer cover broad research areas including but not restricted to endocrinology, neuroscience, physiology, immunology, pathology and cancer.
-
Range of assays available
This list provides an example of the current range of assays available. It does not include the complete list of assays we can or have performed in the past - if you have a query regarding an assay then please contact Mark.
Validated RIA's and ELISA's Assay name Species Sample Origin Recommended volume
for duplicate analysis (µl)6-Sulphatoxymelatonin Human Urine 50 Adiponectin Human Plasma / Serum / Tissue culture media 20 (200 for TC media) Corticosterone Rat / Mouse Plasma / Serum 50 Cortisol Human Saliva 100 Cortisol Human Plasma / Serum / Urine 200 Cortisol Sheep Plasma / Serum 200 Estradiol Human Plasma / Serum / Follicular Fluid 100 Estradiol Pig Plasma / Serum / Follicular Fluid 200 Estrone Human Plasma / Serum 200 Estrone Pig Plasma / Serum 200 Glucagon Rat / Mouse Plasma / Serum 200 Insulin Human Plasma / Serum 200 Insulin Pig Plasma / Serum 200 Insulin Rat / Mouse Plasma / Serum 200 Leptin Rat / Mouse Plasma / Serum 50 Leptin Human Plasma / Serum 100 Luteinising Hormone (LH) Pig Plasma / Serum / Follicular Fluid 400 Melatonin Bovine Milk 400 Melatonin Human Saliva 400 Melatonin Rat / Mouse Plasma / Serum 400 Progesterone Human Plasma / Serum 400 Progesterone Pig Plasma / Serum 200 Prolactin Pig Plasma / Serum 100 COBAS Assay name Species Sample Origin Recommended volume
for duplicate analysis (µl)Adiponectin (Randox) Multi-Species Serum / K2-EDTA plasma 100 Alanine Aminotransferase Multi-species Serum / heparin or EDTA plasma 100 Albumin BCG Multi-species Serum / heparin plasma 100 Alkaline Phosphatase Multi-species Serum / heparin plasma 100 Ammonia Multi-species EDTA plasma only 100 Aspartate Aminotransferase Multi-species Serum / heparin or EDTA plasma 100 Calcium Multi-species Serum / Li-heparin plasma / urine (acidified) 100 Cholesterol Multi-species Serum / Li-heparin or K3-EDTA plasma 100 C-Reactive Protein (Standard and High Sensitivity) Multi-species Serum / plasma: Li-heparin, EDTA, fluoride or citrate 100 Creatinine Multi-species Serum / Li-heparin or EDTA plasma / urine 100 Glucose Multi-species Serum / Li-heparin, EDTA , NaF, K-oxalate plasma / urine / CSF 100 HDL Cholesterol Multi-species Serum / heparin or EDTA plasma 100 Homocysteine Multi-species Serum / heparin or EDTA plasma 100 Iron Multi-species Serum / Li-heparin plasma 100 Lactate Multi-species Na-fluoride, K-oxalate, Na-fluoride/Na-heparin plasma / CSF / (NOT serum) 100 Lactate Dehydrogenase Multi-species Serum / heparin plasma 100 LDL Cholesterol Multi-species Serum / heparin or EDTA plasma 100 Lipase Multi-species Serum / Li-heparin plasma 100 Non-esterified fatty acids / Free fatty acids (Wako) Multi-species Serum 100 Total Protein Multi-species Serum / Li-heparin or K3-EDTA plasma 100 Triglyceride Multi-species Serum / Li-heparin or EDTA plasma 100 Urea / BUN (blood urea nitrogen) Multi-species Serum / Li-heparin, EDTA or fluoride plasma 100 Zinc (Randox) Multi-species Serum / plasma / CSF / urine. No EDTA 200 α-Amylase Multi-species Serum / Li-heparin or EDTA plasma / urine (alkaline) 100
α-Amylase - Pancreatic Multi-species Serum / Li-heparin plasma / urine (alkaline) 100 γ-Glutamyltransferase Multi-species Serum / heparin or EDTA plasma 100
Frequently asked questions
-
What should I do before I start collecting my samples?
-
What type of assay is best?
Over the last few years, ARAF have developed a catalogue of assays that we determined to be the most specific and reliable at measuring the analyte it is supposed to detect. We do not select assays based on the lowest cost, we select them based upon their specificity and reliability, and whether there is an existing body of published literature supporting the use of the product in question. For some analytes, there may be very few choices of assay kits, and for others there are many.
- Where both RIA's and ELISA's are available, the best and most cost effective choice will often be an RIA
- For samples of animal origin, often the only available test for many hormones and other biologically important substances will be an ELISA
- If you are working with small sample volumes, or you are interested in measuring signalling proteins or immune / inflammatory response hormones or proteins, then an ELISA or single or multiplexed Luminex assay may be the most suitable choice for you (the range of possible Luminex assay targets is very large!)
- ELISA's are in general, more restrictive in that with their 96 wells, they have a capacity of only around 38-40 duplicate samples per plate, with the remaining 16-20 duplicate wells taken up by standards and Quality Controls (QC's)
- It's also important to compare the sensitivity of different kits - especially ELISAs and RIAs.
For example, look at the insulin ELISA standard curve shown below (reproduced from a manufacturer protocol). The normal fasting range in a mouse ranges from just over 0.5 up to about 1.6ng/ml. In OD units, this is only a small range: 0.12 units to about 0.27 units. Large, biologically relevant changes in insulin concentration in a sample set could result in only small changes in OD. Accordingly, the precision of the estimates of insulin concentration may be poor - you may end up with poor duplicates and large variation in your sample set results, making repeat tests and larger sample numbers a necessity.
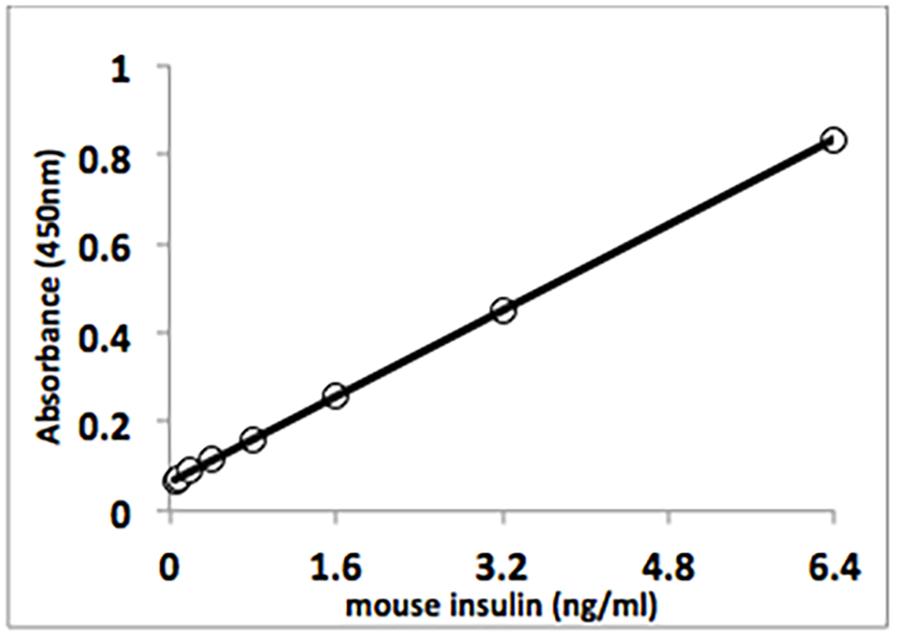
Compare this with the insulin RIA that we use:
The amount of bound tracer changes by ~60% between 0.5 and 1.6ng/ml insulin. In this example, small changes in insulin will result in large shifts in bound tracer. Consequently, for insulin at biologically important concentrations, changes can be determined using the RIA with much greater precision than the equivalent ELISA.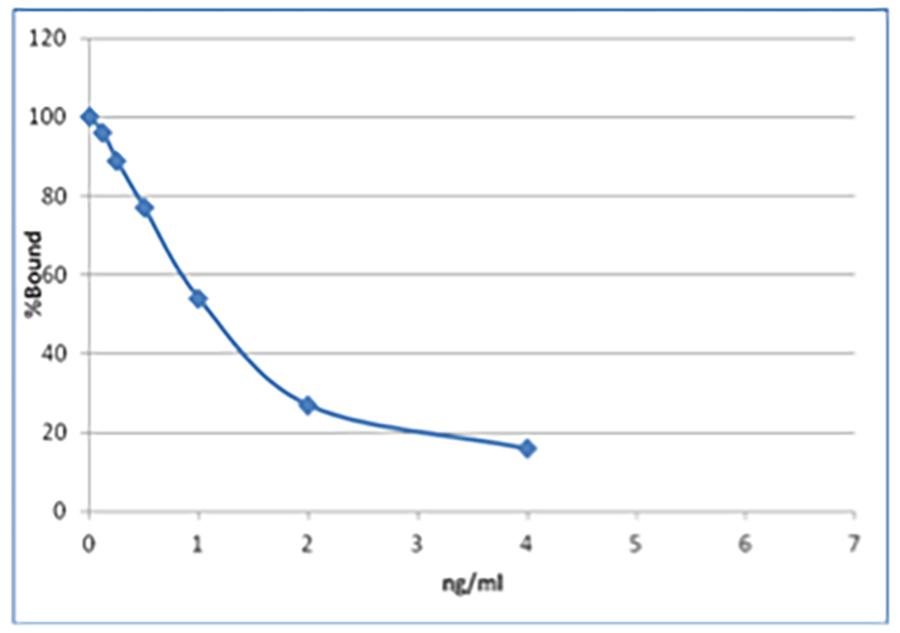
-
I have a custom assay I'd like to run - can ARAF do this?
We have designed custom RIA based assays to detect porcine LH, FSH, Prolactin, and Estrone for clients. Developing a custom assay takes time. If you are interested in a custom assay, we will arrange a time to meet and discuss the proposed project. For the project to succeed, we will need access to supporting evidence (journal references and papers, technical reports etc) as well as a source of an appropriate primary antibody, and an appropriate purified hormone / protein to prepare tracer and standards and then test the new assay.
-
What volume of sample should I provide?
Around 1ml is ideal, to allow for duplicate and repeats analysis on individual samples if necessary. However, we understand that it can be difficult to collect large volumes of plasma from some species (especially mice), so we are more than happy to work with smaller volumes. Be aware however, that smaller sample volumes can reduce an assay's minimum detection limit, making detection of low abundance analytes more difficult. We will look at your list of requested assays, and inform you of how much sample volume you should aim to provide to us per assay.
Many assays are designed with a sample volume of 50µl - 100µl in mind, although some can require as much as 400µl or as little as 10µl.
While duplicates are preferable, if you don't have enough sample volume then assaying samples in single could be a viable option as an alternative to dropping an assay from your study entirely. -
How should I label my samples?
Please label your samples clearly and preferably in two places (ie on the lid and the side, or on the lid and also on the bag, if tubes are individually bagged).
It's best to use a simple labelling system (ie numbering 1-100), and mark the tubes in permanent marker. Please test your marker beforehand and check that it doesn't rub off after contact with water, or after freezing. Permanent markers are not always very permanent!
Sticky printed labels are also ok. However, please check that your tubes will still fit easily when placed in a rack or centrifuge, and that the label is resistant to water exposure as your tubes may get damp with condensation during transport or when stored.
Please send us an Excel spreadsheet with a list of all your samples on it (using the same numbering system as your tubes), and columns for each assay to be performed. -
How should I transport my samples to your facility?
Please address samples:
Attn. Professor David Kennaway
School of Paediatrics & Reproductive Health
Level 3, Medical School South Building
Frome Road
University of Adelaide
SA 5000, Australia
Most major courier services provide an overnight courier service for all major Australian cities. If your samples are packed with ice packs and addressed as described above they should arrive safely at our facility without degradation the next day. Sending samples packed in Dry ice by air is very expensive, but it will help preserve your samples in the event of a transport delay.
Please send your samples early in the week (ie before Wednesday) as this will lessen the chance that they will end up being stored in the courier's central warehouse over a weekend before being forwarded to us.
If you plan on sending samples to us by courier from interstate or overseas, then you should first check with your courier company about their packaging requirements for the transport of biological samples - especially samples of human origin. Please adhere to their requirements, otherwise the courier may either refuse to pick up your samples or deliver them.
Animal or Human derived biological samples that are not known to contain infectious agents are considered Exempt from dangerous goods classification, however certain guidelines still need to be followed when packing samples for transport via courier:- A leak-proof sample container must be used such as a Screw capped tube or Salivette. Try to avoid using Flip-Top tubes if possible, as they tend to spray small amounts of sample around when opened. A foam tray or similar can be used to keep samples upright.
- Please do not over-fill tubes (of any kind). Over filled sample tubes can (and do) burst and break during transit.
- A leak-proof secondary packaging must also be used - A zip locked biological sample bag, plastic lunch box, or screw capped specimen jar. Place some absorbent material inside the container/bag to soak up potential sample leaks during transit.
- A hard outer packaging must be used, such as an esky. Polystyrene foam eskys should be protected by external packaging (ie a cardboard outer box/shell).
- Pack enough pre-chilled cold packs to keep your samples cold during transit (with overnight couriers allow for up to 48 hours). Do Not use ‘wet' ice - it thaws and leaks.
- The courier will require a consignment note to be filled out & attached to the box. They should provide a blank note for filling in.
- If you are based locally in Adelaide, we are more than happy for you to drop samples off in person - see the contact section of this guide for our address, and let us know in advance to expect you. Otherwise, a local same day courier service can be used - just be sure to pack your samples appropriately (ie as above) so they do not get hot or damaged in transit.
-
Package labelling
For biological samples classified as Exempt, put on the outside of your transport box:
- A brief description of the specimens (ie "Exempt human saliva samples")
- The Name and address of sender (not a PO Box number)
- The Name and address of receiver (ie our facility address)
- If the package contains 50mL or more of liquid in total, then use orientation arrows as well.
If you are using dry ice (a Class 9 Dangerous Good) then you MUST notify the courier company, and follow their instructions. Usually:
- They may insist on packing the specimens themselves (or checking your packing).
- They will want to know the net weight (kg) of the dry ice.
- They should provide you with a "Dry Ice" (UN 1845) label and a miscellaneous dangerous goods Class 9 label.
- Ensure your dry ice is packed between the secondary and the outer packaging.
- Ensure ventilation of your package so gas can escape - insulated dry ice transport boxes can be purchased with ventilation holes pre drilled.
-
International shipping
Transporting biological samples from overseas will likely require the use of a specialised sample transportation container, and dry ice to keep your samples chilled. For example, TNT offer a transport system called MedPak Thermo for long distance transport of medical samples.
Follow the couriers' instructions for packing and transport. You will need to obtain a ‘Permit to Import Quarantine Material' from the Australian Commonwealth Quarantine & Inspection Service (AQIS). We can assist in the preparation of the required documentation, so that it can be attached to your consignment of samples. You will need to prepare a detailed manifest listing your samples for attaching to the exterior of the box with the permit documents. -
Timeframe
Once we have a firm written agreement on the number of samples and analytes to be assayed, we will immediately order the needed kits and reagents. Once they arrive, we will assay your samples as soon as practical.
Radioimmunoassays require the use of radioactive tracers for quantitation. These tracers are mostly supplied from overseas, and have very limited shelf lives before decaying (a few weeks only). Thus we cannot keep assay kits or tracer in stock - we must be order them only once we have a firm commitment from a client. Delivery of kits and tracer can take time (4-8 weeks), so please plan your projects accordingly - contact us well in advance if you are working to a deadline (e.g a grant application or an honours project submission date).
We will endeavour to complete the analysis of your samples and get results back to you as quickly as possible. Assays will generally be completed in the order that they arrive. We may be able to negotiate a rapid turnaround with clients who have an urgent request, but due to the supply constraints listed above, we cannot make any guarantees. -
Quality assurance
If we have enough sample volume to work with, we will always repeat samples that fail to satisfy our assay criteria. Repeats are performed if:
- A sample has duplicates with a Coefficient of Variation of greater than 20%.
- A sample measures higher than our highest standard on the assay standard curve, then we will dilute and repeat.
- We will attach a short Quality Control summary to your results spreadsheet, indicating the number of samples assayed, the Reported Means and Expected Means of the QC's, as well as Inter and Intra assay Coefficients of Variation (where applicable).
We will be happy to discuss requests for different statistics or repeat criteria.
-
Quotation process
Quotes are prepared based on the types of assay performed and the total number of samples provided. We use a tiered pricing structure:
- University of Adelaide staff / students - Cost recovery, funds transferred via journal transfer to minimise administrative work.
- External Universities - Largely cost recovery, but a slightly higher fee to take into account the increased administrative workload.
- Commercial entities - Commercial rates apply.
-
Storage sample and return
Upon arrival at our facility, all samples are stored at -20˚C.
Please fill out and sign the section of the client checklist portion of our quotation form regarding sample storage and return. That way, we can be sure of your intentions regarding the disposal or return of your samples, and if samples are to be returned, we can include the cost of sample return in the quote.
Make sure you check your results as soon as possible after you receive them, so that you can contact us if you discover that you would like additional assays performed on those samples before they get returned or discarded!
If you have requested it, samples will be returned soon after results are sent. Please appreciate that we cannot guarantee what the volume of sample will be that is returned to you. If you want to use your samples for other purposes, set aside aliquots before sending your samples to us.
If we are given specific instructions to return your samples, we will follow them. However if we receive no notice of your intentions, our storage facility has limited space and we will discard samples ~1 month after all assays have been completed and finalised results returned to you. -
Results and invoicing
Results and invoices will be sent by email once ready. Please let us know if you would also like paper copies. We can also pre-arrange regular or split invoicing, depending on your needs - ie to allow for payments to be settled prior to the end of a reporting period, or end of financial year etc.
University of Adelaide clients: If debit codes commence "UNIAD", the transaction will be a simple Journal Entry. Nominate a debit code on the attached authority, sign it and return it to David Kennaway.
Non University of Adelaide clients: Must agree to the Standard Terms and Conditions, payment is by cheque to Adelaide Research and Innovation. Please provide the mailing address and ABN of your institution when presenting samples. To ensure funds are available to pay invoices, raise a purchase order prior to sending your samples. -
Acknowledgement of ARAF
ARAF is providing an essential service for users and it is important to recognise the team's contributions to the scientific advancement of the projects. The type of recognition that is most appropriate may be different for individual projects, depending on the contribution that ARAF provides. It is important that ARAF be acknowledged in publications. This might be written as follows:
"The authors gratefully acknowledge the Adelaide Research Assay Facility which provided analysis of ________________." -
What should be included in the 'Materials and Methods' section of my papers?
Upon request, we can provide a short template / guide based upon the assay work we performed for you that can be used for your paper / thesis materials and methods section.
If requesting methods sometime after we have completed your assays, we would appreciate it if you could please give us a quick summary of the type of work done (& dates), so we can quickly look up your records. -
Standard terms and conditions
-
How is our Facility equipped?
Our Facility has been set up specifically with assays in mind.
Radioimmunoassays
We have a high throughput Perkin Elmer 2470 Wizard2 Gamma Counter, capable of analysing 300 I-125 labelled tubes per hour.
ELISA
We have a Tecan Hydroflex plate washer and Biotek Synergy plate reader set up for precise preparation and reading of ELISA plates.
Luminex
The Luminex Assay platform utilises microscopic fluorescent beads covered in covalently bound ligands to permit the assaying of multiple analytes within a 96 well plate format. Ideal for Cancer, Immunology and Cell Signalling applications.
COBAS
The Facility also has a Hitachi COBAS 400 chemical analyser available for measuring small metabolites like glucose and non-esterified free fatty acids.
More information
For more information about ARAF, to discuss your research assay needs or for quotations please contact Mark or David.

Facility Director:
Professor David Kennaway / +61 8 8313 4090
Facility Manager:
Mr Mark Salkeld / +61 8 8313 4410
