Cystic Fibrosis Airway Research Group and Respiratory X-Ray Imaging Laboratory
Our goal is to develop an effective genetic therapy for prevention or treatment of Cystic Fibrosis airway disease.
The group’s research themes are currently focussed on several complementary areas; achieving effective lentiviral CFTR vector gene delivery, lentiviral vector development, upscaling vector production, transducing airway stem cells in situ to enable extended gene expression, developing new delivery methods, and developing rapid and accurate outcome measures for assessment of airway disease using X-ray imaging.
The lentiviral gene therapy we are developing and testing has three clear advantages:
- The only rational way to prevent or halt CF airway disease is by overcoming the fundamental problem – the defective CF gene – by adding a correct copy of the CF gene to airway cells.
- Successful correction will be effective for all CF mutations (of which there are more than 2,000), in contrast to new and expensive drugs that depend on specific CF mutation type, this cure would be suitable for all CF patients.
- Effective gene treatment of airway stem cells – the cells naturally present in the airway that are responsible for repair and renewal of airway tissue – will provide very long-lived correction after the initial dosing is completed, with a goal of seeking lifelong correction for the patient.
The need for fast, reliable, and non-invasive methods to pre-clinically test for correction of CF airway physiological function has led to rapid progress using novel synchrotron X-ray imaging approaches in live mice in collaboration with physicists from Monash University. These techniques quantify the effects of treatments on airway surface function (including mucociliary transit), as well as the local airflow in the lungs during breathing.
Research into translation of methods for potential human application in a non-synchrotron diagnostic setting is continuing in studies performed at the Imaging and Medical Beamline at the Australian Synchrotron, and the Munich Compact Light Source. We now have access to a new National Imaging Facility funded 4Dx Permetium machine located at the SAHMRI Pre-clinical Imagine and Research Laboratory in Adelaide.
Further information:
-
Research
Our achievements
- 1998 First demonstration of an effective pre-treatment to enhance airway gene transfer
- 2001 Airway surface changes detected in CF mice
- 2002 First successful corrective gene transfer in CF mouse airways
- 2005 Gene delivery optimised for maximum effectiveness
- 2008 First demonstration of long-term (>12 month) corrective airway gene transfer in CF mouse airways
- 2008 First synchrotron X-ray imaging of mouse airways to improve measurement of gene transfer success
- 2009 First X-ray detection and measurement of particle movement in live mouse airways
- 2010 Successful translation of airway gene transfer techniques into a large lung (sheep)
- 2010 Optimisation of pre-treatment method for airway gene transfer
- 2011 Synchrotron phase-contrast X-ray imaging reveals fluid dosing dynamics for gene transfer into mouse airways
- 2013 New method for X-ray detection of airway surface changes after treatment
- 2016 New method for assessing regional lung function via X-ray imaging
- 2017 CF rat colony established in Adelaide
- 2018 Ability to bronchoscopically dose gene vector into the lungs of rats
Gene therapy research results
Reporter gene expression
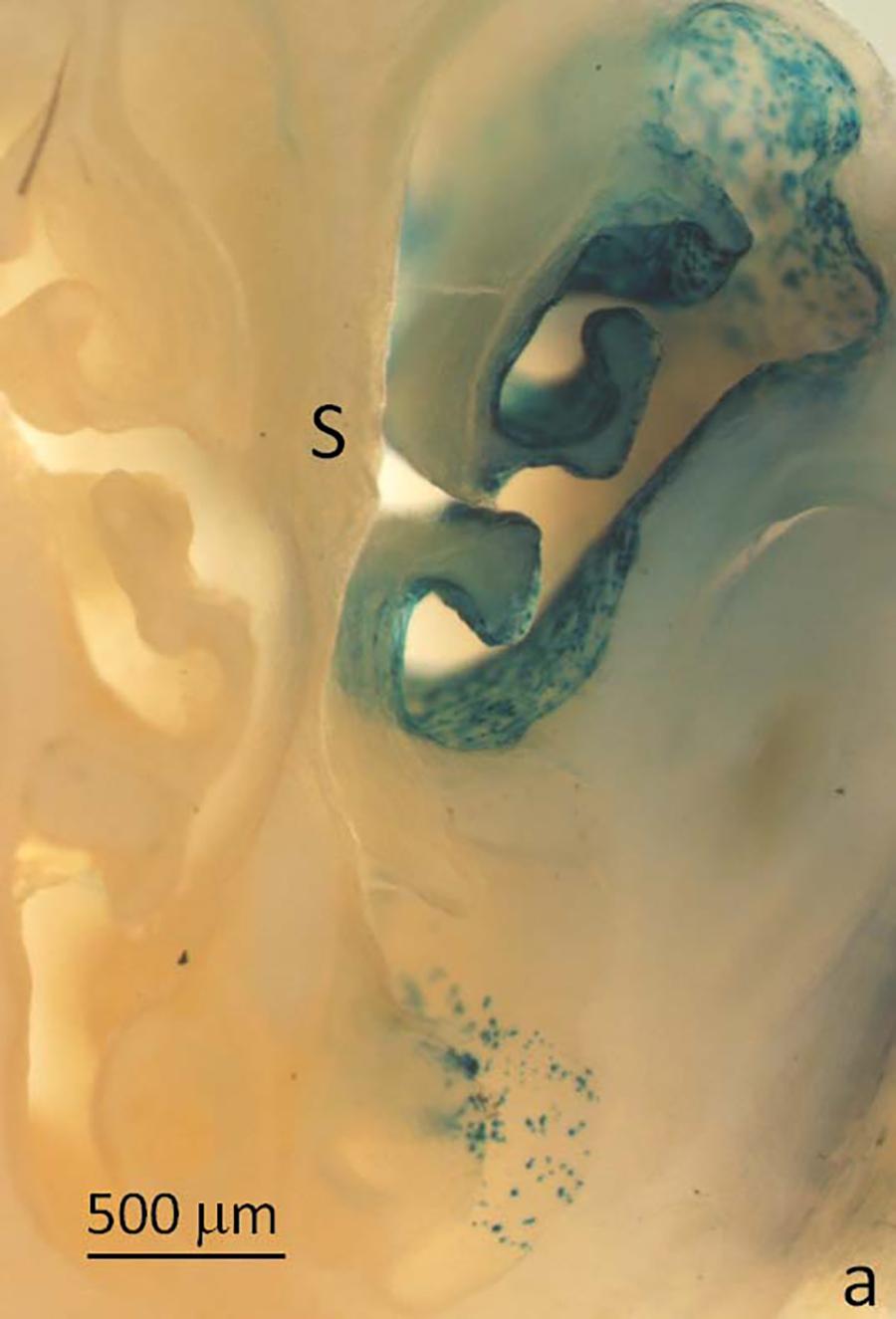
Image of mouse nasal airway LacZ staining (blue cells) on treated (right) side. S =nasal septum. This study demonstrated the efficiency with which our vector can transduce the appropriate cells within the nasal airway of live mice.

This pilot study was designed to determine whether our gene vector delivery protocol is effective in normal ferret lung airways, prior to consideration of studies in CF ferrets. These results verify that our gene vector delivery protocol can produce airway reporter gene transfer in normal ferret trachea. The blue dots are cells that express the LacZ gene. Histology verified that ciliated, non-ciliated and basal cells were transduced in the tracheal epithelium.
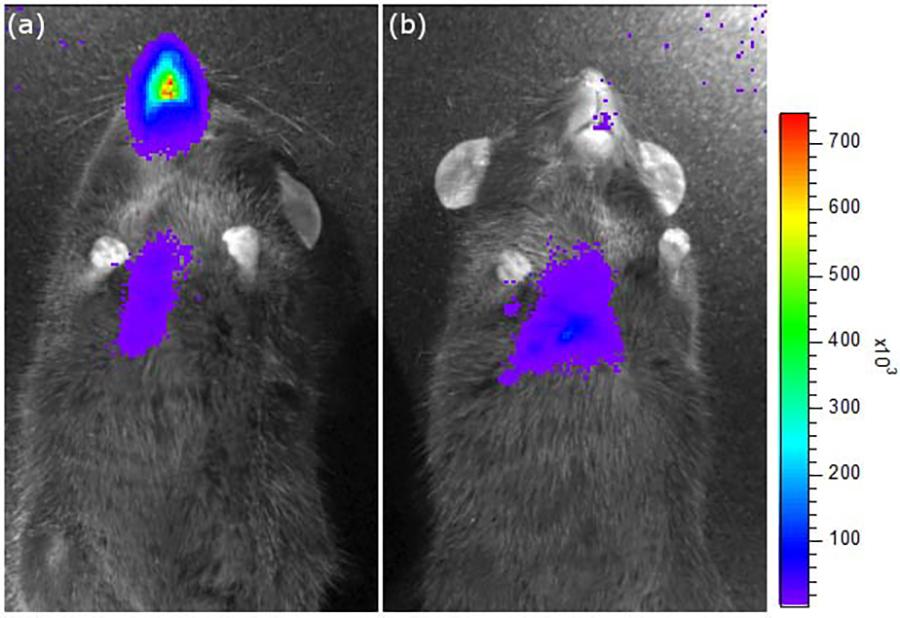
Luciferase gene expression is evident in the nose and lung airways following a nasal administration of our lentiviral vector carrying the luciferase reporter gene. Luciferase gene expression is enhanced in the nasal airways of mice that received a) surface enhancing pre-treatment compared to b) PBS pre-treatment.
Therapeutic CFTR expression
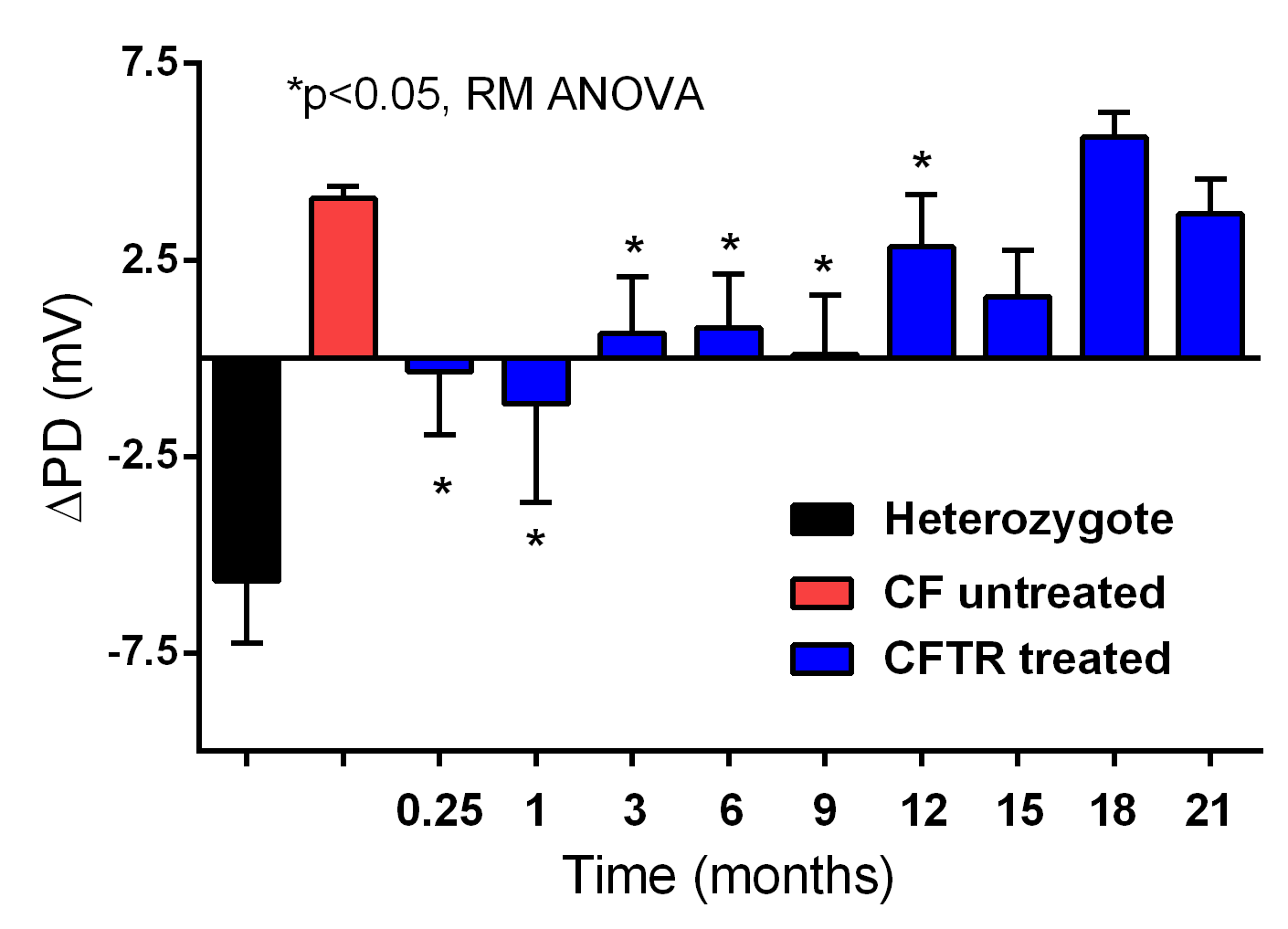
Significant partial correction of the CFTR defect in CF mice was persistent for 12 months following a single treatment with our lentiviral vector carrying the therapeutic CFTR gene. No other group, worldwide, can achieve this longevity of functional expression.
Airway imaging research results
High-resolution imaging

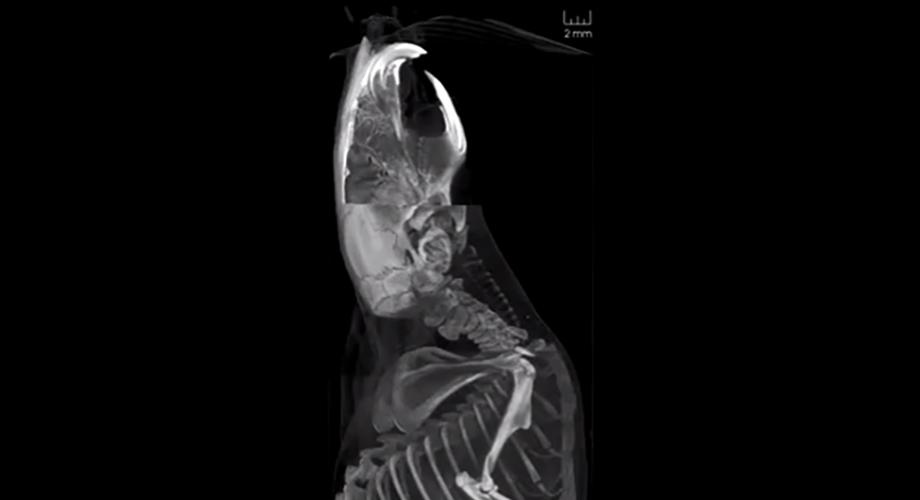
Renderings of high-resolution synchrotron computed tomographic images can be used to visualise microscopic structures within a whole animal. This data was collected during experiments at the SPring-8 Synchrotron.
Mucociliary clearance

Mucociliary clearance in the nose and lungs of live mice can be tracked using synchrotron phase contrast X-ray imaging. Here the effect of hypertonic saline - a clinically used treatment for CF lung disease - can be seen on the clearance of deposited marker particles.
Fluid dosing in the nose and lungs

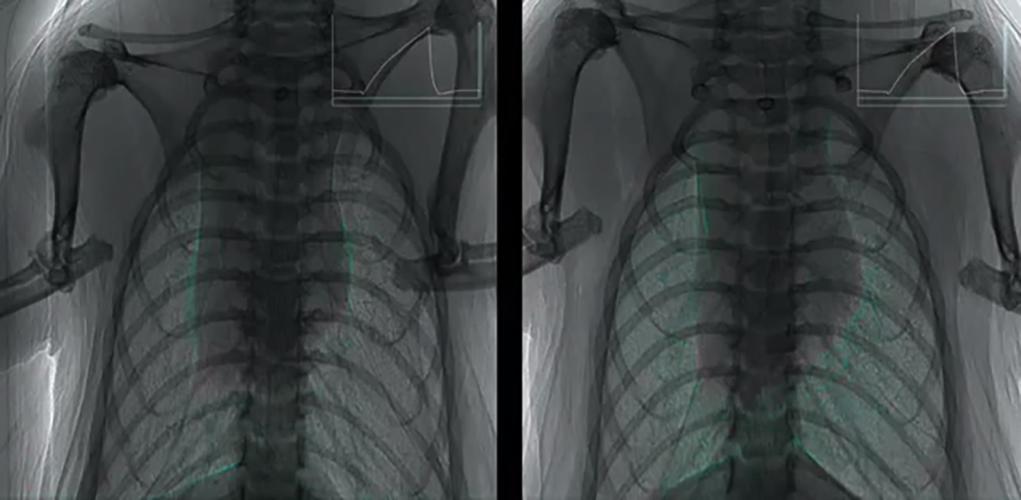
These images show the movement of fluid (a surrogate for our gene vector) in the nose and lungs of live mice.

The group recently performed airway imaging experiments at the recently commissioned Imaging and Medical Beamline at the Australian Synchrotron in Melbourne. Their goal is to develop novel visualisation strategies for respiratory disease. Airways of several animal species were imaged. Here a 3D visualisation of rat anatomy is shown in exquisite detail. Rendering was performed by Dr Anton Maksimenko from the Australian Synchrotron.

Early data from our latest 2012 SPring-8 studies shows a new ability to image the area of mouse nasal airway relevant to future studies for CFTR gene transfer. We can now discern differences in mucociliary clearance following aerosol treatments.
The effects of hypertonic saline and mannitol on clearance are shown.
Latest developments
For respiratory research utilising gene vector delivery to the lung, the size of rodent models has typically necessitated relatively “blind” dosing via the nose, via an endotracheal tube, or through a surgical incision into the trachea. This commonly results in a limited ability to reliably dose specific small regions of lung, and contributes to high levels of transduction variability between animals. We have designed and successfully implemented the first reliable targeted gene vector dosing of small regions in rat lungs using a miniature rigid bronchoscope containing a working channel. This method is now published in Human Gene Therapy methods. An example of bronchoscopic dosing into a CF rat lung is shown above.
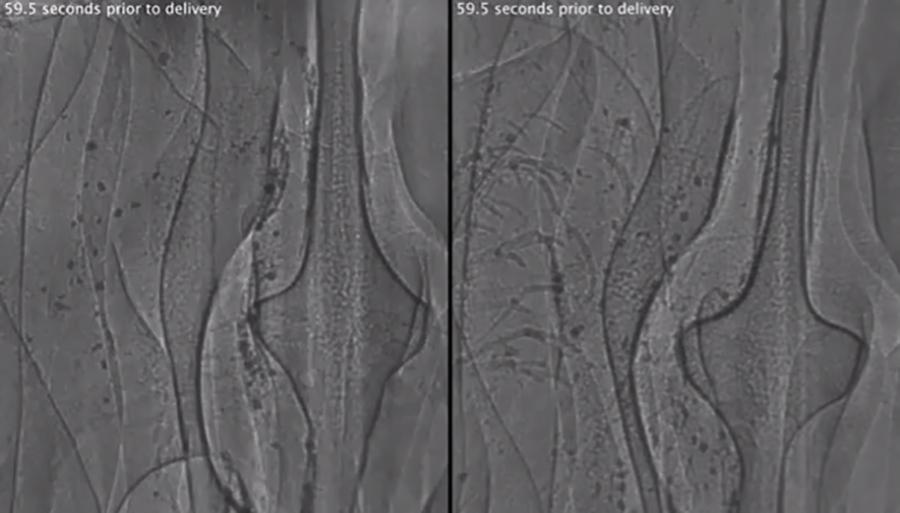
The Australian Synchrotron Imaging and Medical Beamline (IMBL) was designed to be the world’s widest synchrotron x-ray beam, partly to enable clinical imaging and therapeutic applications for humans, as well as for imaging large animal models. We recently performed the first live large animal x-ray phase contrast imaging using this facility. We measured the mucociliary transport behaviour of deposited marker particles in the trachea of live anaesthetised pigs, and in one animal we performed whole-animal high resolution CT. Examples of particles moving along the live pig airway surface are shown above.
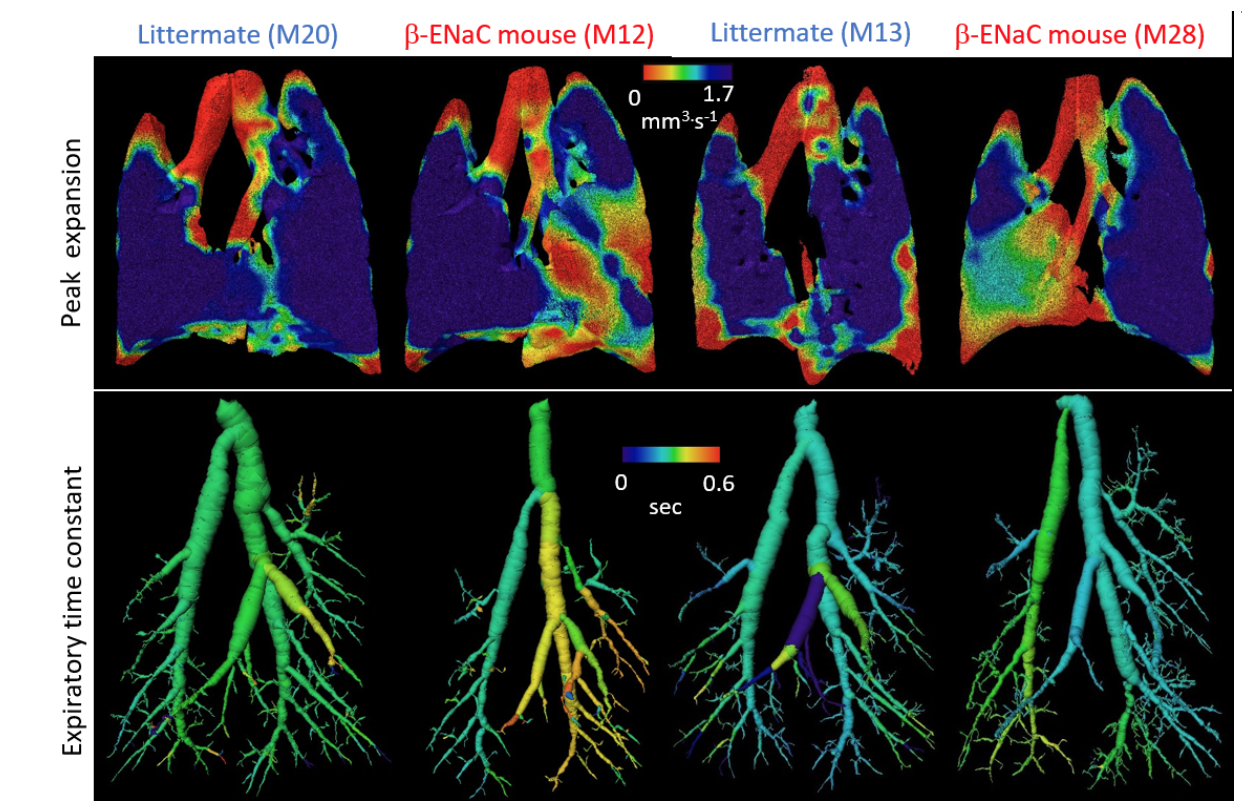
We have previously established a method for examining and quantifying regional lung function deficits in vivo. The process, known as 4DXV combines four-dimensional computed tomography using phase-contrast x-ray imaging with x-ray velocimetry enables us to make local measurements of airflow through the airway tree and regional lung lobes. The image below shows the results from β-ENaC mice, a well-established mode of cystic fibrosis-like disease, and their healthy littermates. We have shown that it is possible to identify the location of lung disease.
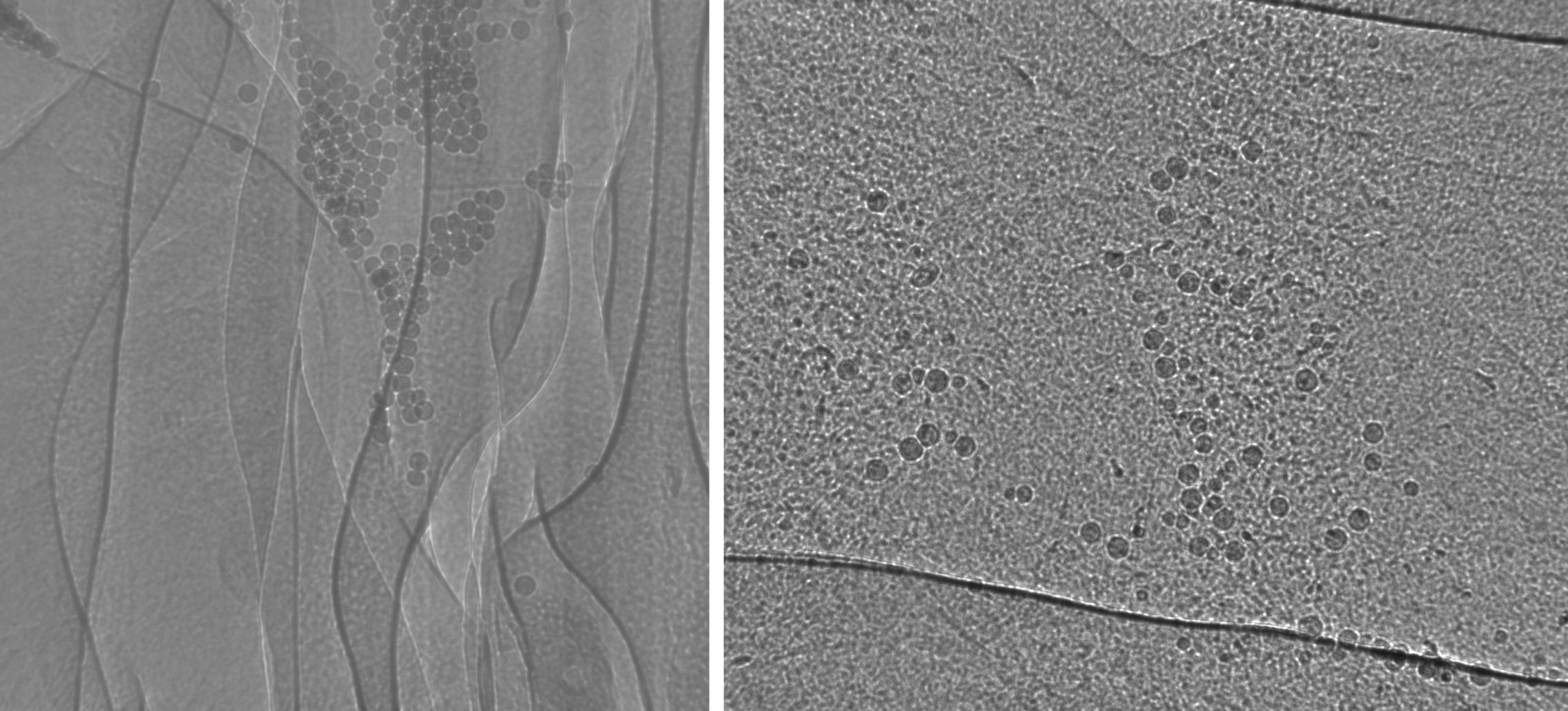
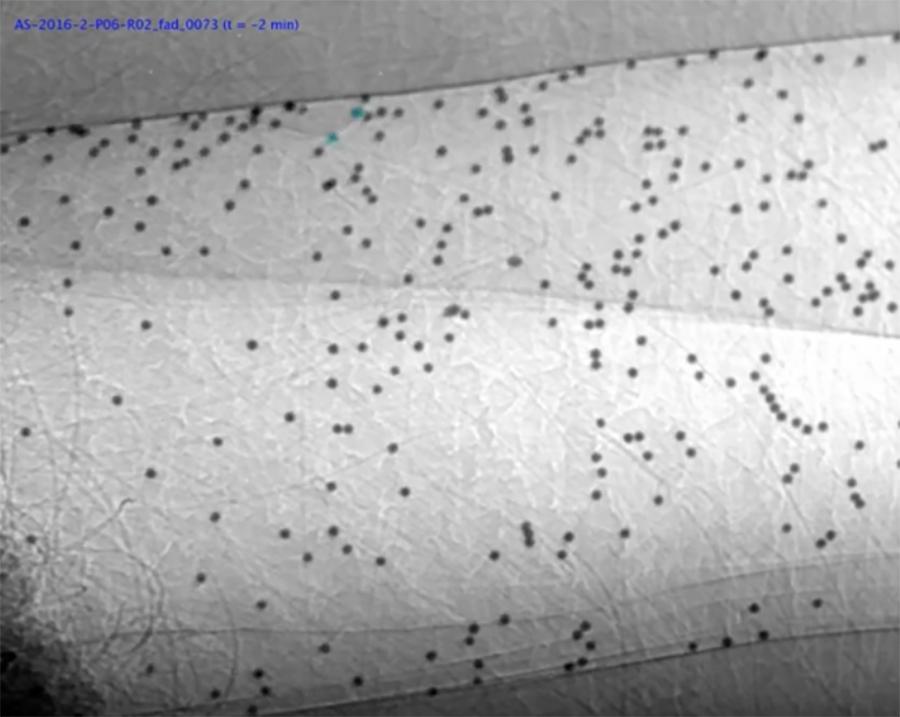
In 2013B SPring-8 studies (left) we have demonstrated the use of glass microspheres as suitable particles for tracking MCT in the nasal airway of CF and normal mice. In 2014A studies (right) we extended this method to the trachea of normal mice. The particles are moved by beating cilia on the airway surface, allowing us to assess the effectiveness of this biological process, and how it changes with treatment. Images ~1.2mm high.

In 2014A studies at SPring-8 Synchrotron in Japan we examined the MCT behaviour of real environmental lead dust (< 50 µm particle size) collected from around Port Pirie. Image is ~1.2 mm high. Here dust can be seen preferentially moving toward the dorsal surface of the trachea. Dust motion is only visible in the moving images due to the phenomenon known as "motion popout"

In 2015-1 studies at the Australian Synchrotron Imaging and Medical Beamline we examined the MCT behaviour of 100 µm particles in segments of excised sheep trachea. Image is ~14 mm high. These studies were performed with a future view to examining MCT in CF pig and CF ferret airways, to quantify the effect of pharmaceutical and genetic therapies in new CF animal models.
-
Patient Story
Meet five-year-old Ella

Ella suffers from cystic fibrosis (CF) and must undergo a daily regimen of physiotherapy to maintain her lung capacity and slow damage to her organs. This includes chest and upper abdomen patting, bubble peps, peri peps, and inhalation of hypertonic saline solution through a ventilated mask.
Ella also takes up to 30 capsules a day containing digestive enzymes that help her body absorb fat every time she has a meal or snack. Her condition brings about recurring chest infections that often require 8-hourly doses of antibiotics for weeks at a time. It is these infections that often lead to lung transplants in CF patients by late adolescence.
Researchers at the Adelaide Cystic Fibrosis Gene Therapy Group are working to help Ella and other children like her by correcting the basic cellular defect that causes CF lung disease. By inserting healthy genes into the defective, disease-causing cells that line the airways, they hope to correct the faulty genetic information that gives rise to respiratory illness.
This technology has the potential to cure CF lung disease and increase the life expectancy of CF sufferers. If successful, it will also help Ella achieve two of her childhood dreams: to become a doctor and learn karate.
We need your help to further this exciting innovation.
Please donate here to make a difference to Ella and the lives of other children with Cystic Fibrosis.
-
Facilities
The Allan Scott CF Research Laboratory

Our 250 m2 CF research laboratory was opened in 2012 at a cost of more than $1.5M, and was funded by a partnership between the Cure4CF Foundation and the Women's and Children's Hospital Foundation. The laboratory is named in memory of the late Allan Scott - who generously donated $500,000 for construction - and is located in the Gilbert Building at the Women's and Children's Hospital within the Department of Respiratory and Sleep Medicine. The laboratory is fully equipped for the design and production of gene vectors, as well as performing all the molecular and histological assays associated with the experiments performed by the group.
The SPring-8 Synchrotron

Many of our X-ray imaging studies demand extremely coherent/monochromatic X-rays that can currently only be produced using a synchrotron, particularly at long beamlines such as the Biomedical Imaging beamlines at the SPring-8 Synchrotron located in Hyogo Prefecture, Japan. Our group typically performs imaging experiments twice a year at SPring-8. Picture: Wikipedia.
The Australian Synchrotron

Picture: Wikipedia.
The Australian Synchrotron Imaging and Medical Beamline (IMBL) was completed early in 2013, and is suitable for some of our X-ray imaging studies. The IMBL offers high-resolution, phase-contrast x-ray imaging of biomedical samples, including live animal imaging. The beamline is 150 metres long, with a satellite building that will later include a medical suite for clinical research as well as extensive support facilities for biomedical and clinical research programs. We have now performed multiple live animal imaging experiments at the IMBL, resulting in a number of publications.
-
Contacts
Who we are

The ReXIL and CFARG team (Left to right): Dr Martin Donnelley, Nikki Reyne, Dr Trish Cmielewski, A/Prof David Parsons, Dr Ali McCarron, Malachi Obst, Bernadette Boog, Dr Nathan Rout-Pitt and Lina Lagerquist
Directors
A/Prof David Parsons
+61 (0)8 816 17004
david.parsons@adelaide.edu.auAssociate Professor David Parsons is the Cystic Fibrosis Airway Research Group (CFARG) Director and Co-Leader of the Respiratory X-ray Lung Imaging Laboratory (ReXIL). He is a Chief Medical Scientist in the Department of Respiratory And Sleep Medicine at the Women's and Children's Hospital, a Robinson Research Institute Research Leader, and an Affiliate of The University of Adelaide Medical School. He has devoted over 25 years to finding a cure for Cystic Fibrosis airway disease, has twice-won Service Excellence Awards, and led the CFARG team to a shared Award for his innovations in respiratory research and testing at the Women's and Children's Hospital. He was recognised as a fellow of the ANZSRS in 2015, and received their research medal in the same year.
Together with his research team, hepioneered a ground-breaking gene delivery technique that has been successful in reversing the basic cellular defect that causes CF in mouse models, showing the clear potential for gene-addition therapy as a long-lasting treatment for CF lung disease.
Identifying the lack of methods to measure success of CF airway gene therapy, David initiated development of novel X-ray imaging techniques able to examine the physiology of airways in living systems, establishing techniques able to measure particle transit and airway surface hydration. Joining with Monash University researchers he helped enable development of X-ray velocimetry techniques to measure lung airflow during breathing in fine detail. Subsequently those researchers commercialised the technology as LVAS (Lung Ventilation Analysis Software LVAS) in a new Australian company, 4DMedical. He is assisting in further development of the technique which is now being used in small and large animals; and in human diagnostics and research, the most recent being a LVAS feasibility study in Adelaide CF and non-CF paediatric patients.
David's creation and development of CF airway gene transfer and of airway imaging techniques, have been supported by the MRFF (a $29M Frontiers grant developing and examining the LVAS technology), as well as the USA CF Foundation, NHMRC, CF Australia, the Cure4CF Foundation, the Fay Fuller Foundation SA, the Gandel Foundation, the Children's Research Fund SA, the Women's and Children's Foundation, and other local funding bodies.
Dr Martin Donnelley
+61 (0)8 816 19181
martin.donnelley@adelaide.edu.auDr Martin Donnelley is a Senior Research Fellow at The University of Adelaide, the Leader of the Respiratory X-ray Imaging Laboratory (ReXIL) and the Co-Director of the CFARG. He trained as a Biomedical Engineer and completed a PhD in Medical Image Processing at Flinders University in 2008. Martin joined the group in 2007 as a post-doctoral researcher on an NHMRC grant.
He has spent the last 15 years developing gene therapy methodologies, as well as technologies for the measurement of in vivo dynamic airway function in animal models. His key achievements lie in the area of non-invasive synchrotron imaging of respiratory processes at scales that have not previously been possible in vivo. He developed a novel non-invasive airway health assessment method based on using synchrotron imaging to track changes in the mucociliary transit (MCT) behaviour of deposited marker particles following pharmaceutical treatments. This work is linked to studies he performed with collaborator Dr Kaye Morgan from Monash University, that show they can also measure changes in airway surface liquid (ASL) depth in live anaesthetised mouse airways. They have now combined these methods into a single powerful airway health assessment method.
In 2016 he and collaborators from Monash University published the first demonstration of the use of X-ray Velocimetry (XV), an X-ray based pulmonary function testing for the quantification of lung disease heterogeneity in B-ENaC mice. This unique and world-leading imaging method gathers lung motion information during normal breathing, and has revolutionary potential since it can detect, quantify and follow changes in regional lung function over time. He now has industry collaborations with 4DMedical (Prof Andreas Fouras) who have commercialised this technology. This work has been funded by the NHMRC, MRFF and other funding bodies.
Postdoctoral Scientists
Dr Trish Cmielewski
+61 (0)8 816 16430
patricia.l.cmielewski@adelaide.edu.auDr Trish Cmielewski gained a BSc in Biology at Flinders University of SA and her early career saw her employed in a wide range of disciplines including Anaesthesia and Intensive Care, Pain Management, Histopathology and Gastroenterology. In 2001 Trish began working as a Medical Scientist with the Department of Respiratory and Sleep Medicine at the Women's and Children's Hospital, Adelaide, South Australia.
Trish has considerable experience in both clinical and experimental aspects of medical research and completed a PhD in lentiviral airway gene therapy for the treatment of cystic fibrosis in a mouse model. Trish's research is currently focused on improving our therapeutic CFTR gene vector into our newly generated rat models of CF. She is also the Manager of the CFARG's Allan Scott Laboratory.
Dr Alexandra McCarron
alexandra.mccarron@adelaide.edu.auDr Ali McCarron joined the Cystic Fibrosis Airway Research Group in 2015, after for her Bachelor of Health Sciences honours project. During her undergraduate Bachelor of Science (Animal Science) studies, Ali become interested in the use of animal models to study human diseases and how such models can play a significant role in the development of novel therapeutic strategies. In 2016 Ali commenced a PhD with the group. The project involved three major components: (1) modification of lentiviral vector production methods, including the use of bioreactor technology, to produce larger quantities of vectors, (2) phenotype characterisation of two novel cystic fibrosis rat models, and (3) development and assessment of airway surface preparation methods for enhancing the efficacy of airway gene transfer. In 2021 Ali received her PhD and qualification and commenced a postdoctoral position with CFARG and ReXIL.
Dr Nathan Rout-Pitt
nathan.rout-pitt@adelaide.edu.auDr Nathan Rout-Pitt completed his PhD at the University of Adelaide in 2015 with a focus on bone disease in the group of lysosomal storage disorders called Mucopolysaccharidoses.
In late 2015, Nathan joined CFARG where he managed the vector production core, producing lentiviral vectors for CFARG and Australia-wide laboratories. During this time he developed and published a method to scale up vector production by as much as 5x. In 2018 Nathan began investigating airway remodelling and fibrosis development in CF lungs. A $75,000 Women's and Children's foundation grant allowed him to begin exploring the alterations in cellular architecture of our Phe508del and 510X CF rat models and their sensitivity to the process of epithelial-mesenchymal transition, which under chronic inflammatory conditions leads to fibrosis.
Nathan briefly left CFARG for a year (2021) to return to lysosomal storage disorders and bone disease, this time looking at Gaucher disease, before returning in 2022. Nathan is continuing to investigate the mechanisms that lead to fibrosis in CF airways and with his expertise in bone is also investigating CF bone disease (CFBD) which typically presents with decreased bone mineral volume and short stature and higher risk of fractures. Decreased trabecular number around the growth plate of long bones and previously reported issues at the hypertrophic zone indicate that there is a need to better understand mechanisms at play in the growth plate causing CFBD, so that we can improve bone health outcomes and better quality of life.
Postgraduate Students
Nikki Reyne
nikki.reyne@adelaide.edu.au
Project: Improving the effectiveness of airway lentiviral gene therapy for cystic fibrosis
Nikki completed a Bachelor of Animal Science at The University of Adelaide, and spent 8 years working in animal laboratories. In 2018 Nikki joined CFARG as a Research Assistant. Her role primary involved overseeing the CF rat colony and assisting with rat procedures. In mid-2019 Nikki started her honours project with the group. Her project optimised nasal potential difference procedures in rats and then assessed CFTR function after delivery of lentiviral vector CFTR to the nose of CF rats. Nikki has continued with the group to undertake a PhD, where she is designing methods to improve the effectiveness of our airway gene therapies, especially in lungs that are infected with bacteria.Lina Lagerquist
lage0006@flinders.edu.au
Project: Detecting respiratory signals in laboratory animals for gated X-ray image acquisition
Lina is in her last semester of a Bachelor of Engineering (Biomedical) at Flinders University. Ever since undertaking school work experience at a hospital she has been interested in medical technology and science. She joined CFARG for her honours project aiming to improve the timing of image acquisition for X-ray imaging experiments in anaesthetised rats. This will help produce high quality images for analysis and quantification of lung tissue damage and airflow in treated and untreated cystic fibrosis rats.Malachi Obst
malachi.obst@student.adelaide.edu.auProject: Investigating the efficacy of gene addition therapy in cystic fibrosis neonatal rats
Malachi completed a Bachelor of Science (Advanced) at the University of Adelaide in 2021. Throughout her studies she found particular interest in genetic diseases, virology and immunology and has joined CFARG for honours this year. The project aims to utilise viral vectors for gene addition therapy and assess the advantages of treating rats during the neonatal stage of life compared to adults.Administrative Staff
Bernadette Boog
+61 (0)8 816 17241
bernadette.boog@adelaide.edu.auBernadette completed a Bachelor of Biomedical Science with Honours through the University of South Australia. The first 15 years of her career saw her gain both diagnostic and research laboratory experience focusing mainly on Immunology and Molecular genetics, before joining CFARG in 2014.
Bernadette is responsible for supporting CFARG and ReXIL as a Research Assistant in the laboratory, as well as providing administrative support and management. As a Research Assistant, Bernadette is involved with both animal and experimental aspects of various projects, and brings continuity to this group of leading edge, globetrotting research scientists. Her administrative role involves management of financial cost centres, coordinating grant funding applications, OGTR, biosafety, ethics annual reports & business plans, and general lab management duties.
Affiliates
Dr Nigel Farrow
+61 (0)8 816 19183
nigel.farrow@adelaide.edu.auDr Nigel Farrow gained a BMSc in molecular biology and genetics from Flinders University, South Australia in 2009 and a BHSc (Hons) from The University of Adelaide School of Medicine in 2010. Nigel joined the Adelaide Cystic Fibrosis Airway Research Group in 2010 for his Honours project, and has remained with the group, completing a PhD in 2014. Nigel has a research interest in gene therapy for cystic fibrosis focusing on endogenous respiratory stem cells and their role in sustained transgene expression. Nigel is also investigating the impact on increasing transgene expression by multiple vector dosing strategies and its impact on the immune system in an animal model. In 2021 Nigel moved to the Weiss Lab at the University of Vermont in the USA to continue his postdoc research program but remains affiliated with CFARG.
Dr Tom Goddard
tom.goddard@sa.gov.auAfter his undergraduate medical degree Dr Tom Goddard worked as an intern and resident medical officer in surgical and medical specialties. He developed a passion for treating and caring for patients with cystic fibrosis as part of his basic physician training. He was employed as a Cystic Fibrosis Fellow at the Women’s and Children’s Hospital in Adelaide before working at the Royal Brompton Hospital, the largest specialty heart and lung centre in the UK. While at the Royal Brompton Hospital he focused on patients with airway issues and performed cardiopulmonary exercise testing and continuous laryngoscopy during exercise. In addition, during the COVID-19 pandemic, he worked in adult intensive care units, providing respiratory expertise on ventilation parameters and performing flexible bronchoscopy for diagnostic and treatment purposes. Dr Goddard returned to the Women’s and Children’s Hospital in June 2020 in a consultant role. He maintains his research interests in cystic fibrosis and provides expertise to the CFARG team, including bronchoscopy for large animal studies. He is also coordinating a paediatric clinical trial of XV imaging in CF and non-CF kids at the WCH. He is an investigator on our new Cystic Fibrosis Foundation studies.
Dr Greg Smith
+61 (0)8 816 17008
greg.smith2@sa.gov.auDr Smith is a clinical respiratory specialist and allergist in the Respiratory and Sleep Medicine Department and part of the CF Clinic team at the WCH. He joined the CFARG Team in 2004, and provides practical experience and advice around the clinical development of CF airway gene therapy and technique training (e.g. bronchoscopic vector delivery). This important experimental-clinical interface assists research to relate to CF patient needs, assisting in the design and analysis of the animal model studies. His input is invaluable as we move towards translation of pre-clinical gene therapy protocols into human clinical trials.
Collaborators
Local
- Prof Robert McLaughlin and Dr Jiawen Li (Uni Adelaide)
- A/Prof Sarah Vreugde and Prof Peter Wormald (Uni Adelaide, TQEH)
- Prof Sandy Hodge and Dr Hai Tran (Uni Adelaide)
- Dr Jimmy Breen (SAHMRI). Assessing lentiviral vector integration sites
- Dr Chris Christou (SAHMRI)
- Dr Ivan Lee and A/Prof Sang-Heon Lee (UniSA)
- A/Prof Albert Juhasz and Dr Euan Smith (UniSA)
- Prof Clive Prestige (UniSA)
- A/Prof Ivanka Prichard (Flinders University)
- Dr Maged Awadalla and Dr Saulo Martelli (Flinders University)
- Dr Emma Parkinson-Lawrence and Prof Sandra Orgeig (UniSA)
National
- Dr Kaye Morgan and Dr Marcus Kitchen (Monash University)
- Dr Chris Hall, Dr Mitzi Klein, Dr Daniel Hausermann and others (IMBL, Australian Synchrotron)
- A/Prof Ivan Bertoncello (University of Melbourne)
- Prof Graeme Zosky (University of Tasmania)
- A/Prof Anthony Kicic (Telethon Institute of Child Health)
- Dr Elena Schneider-Futschik (University of Melbourne)
- A/Prof Deanne Hryciw (Griffith University)
- Dr Shafa Waters (University of New South Wales)
International
- Prof Ric Boucher and Dr Wanda O’Neal (University of North Carolina, Chapel Hill)
- Prof Naoto Yagi, Dr Kentaro Uesugi, Dr Akihisa Takeuchi and other Japanese collaborators (SPring-8 Synchrotron).
- Prof Franz Pfeiffer and Dr Martin Dierolf (Technische Universität München)
- Prof Karl Kunzelmann (Universität Regensburg)
- Prof Dan Weiss (University of Vermont). Stem cell and gene therapy studies for CF
- Prof Nadia Ameen (Yale University)
-
Publications
-
A. McCarron, P. Cmielewski, V. Drysdale, D. Parsons, M. Donnelley, “Effective viral-mediated lung gene therapy: Is airway surface preparation necessary?”, Gene Therapy, 2022.
-
D. Hryciw, C. Jackson, N. Shrestha, D. Parsons, M. Donnelley, A. McAinch, “Role for animal models in understanding essential fatty acid deficiency in cystic fibrosis”, Cellular and Molecular Life Sciences, 2021.
-
N. Rout-Pitt, M. Donnelley, D. Parsons, “In-vitro optimisation of Miniature Bronchoscope Lentiviral Vector Delivery for the Small Animal Lung”, Experimental Lung Research, 2021.
-
P. Cmielewski, J. Delhove, M. Donnelley, D. Parsons, “Assessment of lentiviral vector mediated CFTR correction in mice using an improved rapid in vivo nasal potential difference measurement protocol”, Frontiers in Pharmacology, section Advances in Cell-Based and Gene-Based Therapies for Respiratory Diseases, 2021.
-
N. Reyne, P. Cmielewski, A. McCarron, J. Delhove, D. Parsons, M. Donnelley, “Single-dose lentiviral mediated gene therapy recovers CFTR function in cystic fibrosis knockout rats”, Frontiers in Pharmacology, section Advances in Cell-Based and Gene-Based Therapies for Respiratory Diseases, 2021.
-
C. Carpentieri, N. Farrow, P. Cmielewski, N. Rout-Pitt, A. McCarron, E. Knight, D. Parsons, M. Donnelley, “The effects of conditioning and lentiviral vector pseudotype on short and long-term airway reporter gene expression in mice”, Human Gene Therapy, 2021.
-
A. McCarron, N. Farrow, P. Cmielewski, E. Knight, M. Donnelley, D. Parsons, “Breaching the delivery barrier: Chemical and physical airway epithelium disruption strategies for enhancing lentiviral-mediated gene therapy”, Frontiers in Pharmacology, section Advances in Cell-Based and Gene-Based Therapies for Respiratory Diseases, 2021.
-
K. Allan, N. Farrow, M. Donnelley, A. Jaffe, S. Waters, “Treatment of Cystic Fibrosis: From Gene- to Cell-Based Therapies”, Frontiers in Pharmacology, section Advances in Cell-Based and Gene-Based Therapies for Respiratory Diseases, 2021.
-
N. Farrow, P. Cmielewski, J. Delhove, N. Rout-Pitt, L. Vaughan, T. Kuchel, C. Christou, J. Finnie, M. Smith, E. Knight, M. Donnelley, D. Parsons, “Towards human translation of lentiviral airway gene delivery for cystic fibrosis: A one-month CFTR and reporter gene study in marmosets”, Human Gene Therapy, 2021.
-
A. McCarron, D. Parsons, M. Donnelley, “Animal and Cell Models for Cystic Fibrosis: Which Model is Right for Your Application?”, American Journal of Pathology, vol. 191(2), pp. 228-242, 2021.
-
D. Parsons, M. Donnelley, “Will airway gene therapy for cystic fibrosis improve lung function? New imaging technologies can help us find out”, Human Gene Therapy, vol. 31(17-18), pp. 973-984, 2020.
-
F. Werdiger, K. Morgan, M. Donnelley, S. Dubsky, R. Murrie, R. Carnibella, C. Samarage, Y. How, G. Zosky, A. Fouras, D. Parsons, "Quantification of muco-obstructive lung disease variability in mice via laboratory X-ray velocimetry", Scientific Reports, vol. 10(10859), 2020.
-
H. Tran, M. Macowan, A. Abdo, M. Donnelley, D. Parsons, S. Hodge, “Enhanced inflammasome activation and reduced sphingosine-1 phosphate S1P signalling in a respiratory mucoobstructive disease model”, Journal of Inflammation, vol. 17(16), 2020.
-
M. Gardner, D. Parsons, K. Morgan, A. McCarron, P. Cmielewski, R. Gradl, M. Donnelley, “Towards automated in vivo mucociliary transport measurement: Detecting and tracking particle movement in synchrotron phase contrast X-ray images”, Physics in Medicine and Biology, vol. 65(14), 2020.
-
A. McCarron, P. Cmielewski, N. Reyne, C. McIntyre, J. Finnie, F. Craig, N. Rout-Pitt, J. Delhove, J. Schjenken, H. Chan, B. Boog, E. Knight, R. Gilmore, W. O'Neal, R. Boucher, D. Parsons, M. Donnelley, “Phenotypic characterisation and comparison of Phe508del and CFTR knockout rat models of cystic fibrosis generated by CRISPR/Cas9 gene editing”, American Journal of Pathology, vol. 190(5), pp. 977-993, 2020.
-
J. Delhove, I. Osenk, I. Prichard, M. Donnelley, “Acceptability of gene therapy: A systematic review”, Human Gene Therapy, Accepted for publication, 2019.
-
R. Murrie, F. Werdiger, M. Donnelley, Y. Lin, R. Carnibella, C. Samarage, I. Pinar, M. Preissner, J. Wang, J. Li, K. Morgan, D. Parsons, S. Dubsky, A. Fouras, “Real time in-vivo imaging of regional lung function in a mouse model of cystic fibrosis on a laboratory x-ray source”, Scientific Reports, Accepted for publication, 2019.
-
K. Morgan, D. Parsons, P. Cmielewski, A. McCarron, R. Gradl, N. Farrow, K. Siu, A. Takeuchi, Y. Suzuki, K. Uesugi, M Uesugi, N. Yagi, C. Hall, M. Klein, A. Maksimenko, A. Stevenson, D. Hausermann, M. Dierolf, F. Pfeiffer, M. Donnelley, “Methods for dynamic synchrotron X-ray respiratory imaging of live animals”, Journal of Synchrotron Radiation, vol. 27, pp. 164-75, 2019.
-
K. Morgan, R. Gradl, M. Dierolf, C. Jud, B. Günther, F. Werdiger, M. Gardner, P. Cmielewski, A. McCarron, N. Farrow, H. Haas, M. Kimm, L. Yang, D. Kutschke, T. Stoeger, O. Schmid, K. Achterhold, F. Pfeiffer, D. Parsons, M. Donnelley, “In vivo x-ray imaging of the respiratory system at conventional and compact synchrotrons”, Proc. SPIE 11113, Developments in X-Ray Tomography XII, 111130G, 2019.
-
L. Yang, R. Gradl, M. Dierolf, W. Möller, D. Kutschke, A. Feuchtinger, L. Hehn, M. Donnelley, B. Günther, K. Achterhold, A. Walch, T. Stoeger, D. Razansky, P. Pfeiffer, K. Morgan,O. Schmid, “Multimodal precision imaging of pulmonary nanoparticle delivery in mice: Dynamics of application, spatial distribution, and dosimetry”, Small, vol. 15(49), 2019.
-
F. Kastury, E. Smith, E. Lombi, M. Donnelley, P. Cmielewski, D. Parsons, K. Scheckel, A. Kingston, G. Myers, D. Paterson, M. de Jonge, A. Juhasz, “Dynamics of lead bioavailability in indoor dust and spectroscopic investigation of the link between ingestion and inhalation pathways”, Environmental Science & Technology, vol. 53(19), pp. 11486-11495, 2019.
-
S. Kedzior, T. Bianco-Miotto, J. Breen, K. Diener, M. Donnelley, K. Dunning, M. Penno, A. Rumbold, J. Schjenken, D. Sharkey, N. Hodyl, T. Fullston, M. Gardiner, H. Brown, “It takes a community to conceive: An analysis of the scope, nature and accuracy of online sources of health information for couples trying to conceive”, Reproductive Biomedicine and Society Online, Accepted for publication, 2019.
-
F. Kastury, E. Smith, E. Doelsh, E. Lombi, M. Donnelley, P. Cmielewski, D. Parsons, K. Scheckel, D. Paterson, M. de Jonge, C. Herde, A. Juhasz, “In-vitro, in-vivo and spectroscopic assessment of lead exposure reduction via ingestion and inhalation pathways following immobilization using phosphorus and iron amendments”, Environmental Science & Technology, vol. 53(17), pp. 10329-41, 2019.
-
M. Gardner, A. McCarron, K. Morgan, D. Parsons, M. Donnelley, “Particle coating alters mucociliary transit in excised rat trachea: A synchrotron X-ray imaging study”, Scientific Reports, vol. 9, 10983, 2019.
-
R. Gradl, M. Dierolf, L. Hehn, B. Günther, W. Möller, D. Kutschke, L. Yang, T. Stoeger, B. Gleich, K. Achterhold, M. Donnelley, F. Pfeiffer, O. Schmid, K. Morgan, “Visualising respiratory treatment delivery and deposition using x-ray imaging”, Journal of Controlled Release, vol. 307, pp. 282-291, 2019.
-
A. McCarron, M. Donnelley, McIntyre, D. Parsons, “Transient lentiviral vector production using a packed-bed bioreactor system”, Human Gene Therapy Methods, vol. 30(3), 2019.
-
H. Jung, S. Lee, M. Donnelley, D. Parsons, I. Lee, V. Stamatescu, “Multiple marker particle tracking in Synchrotron time-lapse X-ray images for assessment of mucociliary clearance in live mouse nasal airways”, Pattern Recognition, vol. 93, pp. 485-497, 2019.
-
Z. Wang, J. Cai, W. Guo, M. Donnelley, D. Parsons, I. Lee, “Backprojection Wiener Deconvolution for Computed Tomographic Reconstruction”, PLOS ONE, 13(12): e0207907, 2018.
-
M. Donnelley, D. Parsons, “Gene therapy for cystic fibrosis lung disease: Overcoming the barriers to translation to the clinic”, Frontiers in Pharmacology, section Pharmacology of Ion Channels and Channelopathies, vol. 9(1381), 2018.
-
M. Donnelley, M. Klein, D. Hausermann, C. Hall, A. Maksimenko, K. Morgan, D. Parsons, “Live pig airway surface imaging and whole-animal CT at the Australian Synchrotron Imaging and Medical Beamline”, Journal of Synchrotron Radiation, vol. 26, 2018.
-
R. Gradl, M. Dierolf, L Hehn, B. Gunther, D. Kutschke, L. Yang, W. Moller, T. Stoeger, M. Kimm, H. Haas, N. Roiser, D. Pfeiffer, M. Donnelley, C. Jud, B. Gleich, D. Parsons, K. Achterold, O. Schmid, F. Pfeiffer, K. Morgan, “Dynamic X-ray Imaging at the Munich Compact Light Source”, Proceedings of Microscopy & Microanalysis, vol. 24(s2), pp. 352-353, 2018.
-
N. Rout-Pitt, N. Farrow, D. Parsons, M. Donnelley, “Epithelial-Mesenchymal Transition (EMT): A universal process in lung diseases with implications for cystic fibrosis patients”, Respiratory Research, 2018.
-
C. McIntyre, M. Donnelley, N. Rout-Pitt, D. Parsons, “Lobe-specific gene vector delivery to rat lungs using a miniature bronchoscope”, Human Gene Therapy, Accepted for publication, 2018.
-
N. Farrow, P. Cmielewski, M. Donnelley, N. Rout-Pitt, Y. Moodley, I. Bertoncello, D. Parsons, “Epithelial disruption: A new paradigm enabling human airway stem cell transplantation”, Stem Cell Research and Therapy, Accepted for publication, 2018.
-
R. Gradl, M. Dierolf, B. Gunther, L. Hehn, W. Moller, D. Kutscheke, L. Yan, M. Donnelley, R. Murrie, A. Erl, T. Stoeger, B. Gleich, K. Achterhold, O. Schmid, F. Pfeiffer, K. Morgan, “In-vivo Dynamic Phase-Contrast X-ray Imaging using a Compact Light Source”, Scientific Reports, vol. 8, 6788, 2018.
-
A. McCarron, D. Parsons, M. Donnelley, “Airway disease phenotypes in animal models of cystic fibrosis”, Respiratory Research, vol. 19:54, 2018.
-
N. Rout-Pitt, A. McCarron, C. McIntyre, M. Donnelley, D. Parsons, “Upscaling the production of a VSV-G pseudotyped lentiviral vector using cell factories”, Journal of Biological Methods, vol. 5(2):e90, 2018.
-
N. Farrow, M. Donnelley, P. Cmielewski, N. Rout-Pitt, C. McIntyre, I. Bertoncello, D. Parsons, “The role of basal cells in producing persistent lentivirus-mediated airway gene expression”, Human Gene Therapy, vol. 29(6), pp. 653-662, 2018.
-
A. McCarron, M. Donnelley, D. Parsons, “Scale-up of lentiviral vectors for gene therapy: advances and challenges”, Cell and Gene Therapy Insights, 2017.
-
P. Cmielewski, N.Farrow, S. Devereux, D. Parsons, M. Donnelley, “Gene therapy for Cystic Fibrosis: Improved delivery techniques and conditioning with lysophosphatidylcholine enhance lentiviral gene transfer in mouse lung airways”, Experimental Lung Research, vol. 43(9-10), pp. 426-433, 2017.
-
M. Donnelley, M. Awadalla, K. Morgan, N. Farrow, C. Hall, D. Parsons, “Measuring mucociliary clearance activity in live excised large animal trachea at the Australian Synchrotron Imaging and Medical Beamline”, Respiratory Research, vol. 18:95, 2017.
-
H. Jung, I. Lee, S. Lee, D. Parsons, M. Donnelley, “Multiple mucociliary transit marker tracking in synchrotron X-ray images using the global nearest neighbour method”, IEEE Conference Proceedings, Accepted for publication, 2017.
-
H. Jung, I. Lee, S. Lee, M. Donnelley, D. Parsons, “Automated detection of circular marker particles in synchrotron phase contrast x-ray images of live mouse nasal airways for cystic fibrosis therapy assessment", Expert Systems with Applications, vol. 73, pp. 57-68, 2017.
-
A. McCarron, M. Donnelley, C. McIntyre, D. Parsons, "Challenges of up-scaling lentivirus production and processing”, Journal of Biotechnology, vol. 240, pp. 23-30[MD1] , 2016.
-
K. Morgan, T. Petersen, M. Donnelley, N. Farrow, D. Parsons, D. Paganin, “Capturing and visualizing transient x-ray topological features by single-grid phase imaging”, Optics Express, vol. 24(21) pp. 24435-24450, 2016.
-
M. Donnelley, K. Morgan, N. Farrow, R. Carnibella, R. Murrie, A. Fouras, D. Parsons, “Alteration of Mouse Nasal Airway Surface Mucociliary Transit by Airway Rehydrating Agents”, SPring-8 Research Reports, Accepted for publication, 2016.
-
C. Stahr, C. Samarage, D. Parsons, M. Donnelley, N. Farrow, K. Morgan, G. Zosky, R. Boucher, K. Siu, S. Dubsky, A, Fouras, “Quantification of heterogeneity in lung disease with image-based pulmonary function testing”, Scientific Reports, vol. 6, 2016.
-
M. Donnelley, K. Morgan, K. Siu, N. Farrow, D. Parsons, “Non-Invasive Airway Health Measurement Using Synchrotron X-Ray Microscopy of High Refractive Index Glass Microbeads”, AIP Conf. Proc. vol. 1696, 020011, 2016.
-
R. Murrie, K. Morgan, A. Maksimenko, A. Fouras, D. Paganin, C. Hall, K. Siu, D. Parsons, M. Donnelley, Live small animal x-ray lung velocimetry and lung micro-tomography at the Australian Synchrotron Imaging and Medical Beamline, Journal of Synchrotron Radiation, vol. 22, 2015.
-
Cmielewski, P., Donnelley, M. & Parsons, D.W. Long-term therapeutic and reporter gene expression in lentiviral vector treated cystic fibrosis mice. J Gene Med 16, 291-299 2014.
-
P. Cmielewski, M. Donnelley, N. Farrow, C. McIntyre, J. Penny-Dimri, T. Kuchel, D. Parsons, Transduction of Ferret Airway Epithelia by a Lentiviral Gene Vector, BMC Pulmonary Medicine 14, 183 2014.
-
K. Morgan, M. Donnelley, N. Farrow, A. Fouras, R. Boucher, N. Yagi, Y. Suzuki, A. Takeuchi, K. Uesugi, K. Siu, D. Parsons, In vivo fertilisation of airway surface liquid: monitoring hydration improvement in the assessment of CF treatments, American Journal of Respiratory and Critical Care Medicine, Vol. 190, pp 469-472, 2014.
-
M. Donnelley, K. Morgan, K. Siu, A. Fouras, N. Farrow, R. Carnibella, D. Parsons, Tracking extended mucociliary transport activity of individual deposited particles: Longitudinal synchrotron imaging in live mice, Journal of Synchrotron Radiation, Vol. 21, (2014).
-
M. Donnelley, K. Morgan, K. Siu, N. Farrow, C. Stahr, R. Boucher, A. Fouras, D. Parsons, Non-invasive airway health assessment: Synchrotron imaging reveals effects of rehydrating treatments on mucociliary transit in-vivo, Scientific Reports, 4, 3689, (2014).
-
Morgan, K.S., Donnelley, M., Paganin, D.M., Fouras, A., Yagi, N., Suzuki, Y., Takeuchi, A., Uesugi, K., Boucher, R.C., Parsons, D.W. & Siu, K.K. Measuring airway surface liquid depth in ex vivo mouse airways by x-ray imaging for the assessment of cystic fibrosis airway therapies, PLoS One 8, e55822 (2013).
-
Farrow, N., Miller, D., Cmielewski, P., Donnelley, M., Bright, R. & Parsons, D.W. Airway gene transfer in a non-human primate: Lentiviral gene expression in marmoset lungs, Scientific Reports 3, 1287 (2013).
-
Donnelley, M., Morgan, K.S., Siu, K.K. & Parsons, D.W. Variability of In Vivo Fluid Dose Distribution in Mouse Airways Is Visualized by High-Speed Synchrotron X-Ray Imaging, Journal of Aerosol Medicine and Pulmonary Drug Delivery (2013).
-
Morgan, K.S., Paganin, D.M., Parsons, D.W., Donnelley, M., Yagi, N., Uesugi, K., Suzuki, Y., Takeuchi, A. & Siu, K.K.W. Single Grating X-Ray Imaging For Dynamic Biological Systems. International Workshop on X-Ray and Neutron Phase Imaging with Gratings 1466, 124-129 (2012).
-
Donnelley, M., Siu, K.K., Jamison, R.A. & Parsons, D.W. Synchrotron phase-contrast X-ray imaging reveals fluid dosing dynamics for gene transfer into mouse airways, Gene Therapy 19, 8-14 (2012).
-
Donnelley, M., Morgan, K., Siu, K.K.W. & Parsons, D.W. Dry deposition of pollutant and marker particles onto live mouse airway surfaces enhances monitoring of individual particle mucociliary transit behaviour, Journal of Synchrotron Radiation 19, 551-558 (2012).
-
Liu, C., Wong, E., Miller, D., Smith, G., Anson, D. & Parsons, D. Lentiviral airway gene transfer in lungs of mice and sheep: successes and challenges, Journal of Gene Medicine 12, 647-658 (2010).
-
Donnelley, M., Parsons, D., Morgan, K. & Siu, K. Animals In Synchrotrons: Overcoming Challenges For High-Resolution, Live, Small-Animal Imaging. 6th International Conference on Medical Applications of Synchrotron Radiation 1266, 30-34 (2010).
-
Donnelley, M., Parsons, D., Morgan, K. & Siu, K. Animals In Synchrotrons: Overcoming Challenges For High-Resolution, Live, Small-Animal Imaging. AIP Proceedings 1266, 30-34 (2010).
-
Donnelley, M., Morgan, K., Skinner, W., Suzuki, Y., Takeuchi, A., Uesugi, K., Yagi, N., Siu, K. & Parsons, D. A new technique to examine individual particle and fibre deposition and transit behaviour on live mouse trachea, Journal of Synchrotron Radiation 17, 719-729 (2010).
-
Cmielewski, P., Anson, D.S. & Parsons, D.W. Lysophosphatidylcholine as an adjuvant for lentiviral vector mediated gene transfer to airway epithelium: effect of acyl chain length, Respiratory Research 11, 84 (2010).
-
Stocker, A.G., Kremer, K.L., Koldej, R., Miller, D.S., Anson, D.S. & Parsons, D.W. Single-dose lentiviral gene transfer for lifetime airway gene expression, Journal of Gene Medicine 11, 861-867 (2009).
-
Morgan, K., Paganin, D., Parsons, D., Donnelley, M., Yagi, N., Uesugi, K., Suzuki, Y., Takeuchi, A. & Siu, K. Optimising Coherence Properties for Phase Contrast X-Ray Imaging (PCXI) to Reveal Airway Surface Liquid (ASL) as an Airway Health Measure. IFMBE Proceedings, World Congress on Medical Physics and Biomedical Engineering 25/II, 135-138 (2009).
-
Donnelley, M., Morgan, K.S., Fouras, A., Skinner, W., Uesugi, K., Yagi, N., Siu, K.K.W. & Parsons, D.W. Real-time non-invasive detection of inhalable particulates delivered into live mouse airways, Journal of Synchrotron Radiation 16, 553-561 (2009).
Recent conference posters
2022
American Society of Gene and Cell Therapy - Online
- Donnelley et al., “In Vivo Lentiviral Gene Transfer to Airway Surfaces for Cystic Fibrosis is Improved by Magnetic Guidance of Particles”
- Drysdale et al., “Miniature Devices for Controlled Airway Surface Perturbation in Rats: Which Device Produces the Best Lentiviral Vector Gene Transfer?”
2021
American Society of Gene and Cell Therapy - Online
- Reyne et al., “Lentiviral airway gene therapy correction of CFTR function in knockout cystic fibrosis rats”
North American Cystic Fibrosis Conference - Online
- Farrow et al., “Neonatal Airway Gene Therapy Delivery to Enable Effective Adult Dosing: A Lentiviral Vector Study”
- Donnelley et al., “Phe508del and knockout cystic fibrosis rat lung phenotype assessment via flexiVent and x-ray velocimetry”
2020
American Society of Gene and Cell Therapy - Online
- Rout-Pitt et al, Development of an Epithelial Mesenchymal Transition Tracing Vector
- Carpentieri et al., Assessing lentiviral vector multiple re-dosing schedules for improving and sustaining transgene expression
North American Cystic Fibrosis Conference - Online
- Carpentieri et al., Acute Pseudomonas aeruginosa airway infection models in wild-type and Phe508del cystic fibrosis rats
- McCarron et al., Airway gene-addition therapy for CF lung disease: strategies for improving gene transfer efficacy and longevity
- Reyne et al., Measuring CFTR function in CF rats: Optimisation of the nasal potential difference technique
2019
North American Cystic Fibrosis Conference - Nashville, USA
- Werdiger et al., Quantification of local lung disease in rat models of cystic fibrosis disease
- Farrow et al., Assessing lentiviral vector re-dosing schedules for improving and sustaining transgene expression
- McCarron et al., Phenotype characterisation of Phe508del and CFTR knockout cystic fibrosis rats
Australasian Cystic Fibrosis Conference - Perth
Thoracic Society of Australia and New Zealand - Gold Cost
- Cmielewski et al., Phenotype characterisation of two CF rat models: an update
- Rout-Pitt et al., Optimisation of Bronchoscopic Gene Vector Delivery for Direct Lobe Targeting in Rat Lungs
- Macowan et al., Dysregulated S1P Signalling in a Mouse Model of Cystic Fibrosis-like Lung Disease Produced by β-ENaC Overexpression
American Society of Gene and Cell Therapy - Washington, USA
- Donnelley et al., A systematic review of the public acceptability of gene therapy and gene editing for human applications
- McCarron et al., Transient lentiviral vector production using a packed-bed bioreactor system
2018
North American Cystic Fibrosis Conference - Denver, USA
- Werdiger et al., Detection of regional lung disease in B-ENaC mice on a laboratory X-ray source
- Cmielewski et al., Phenotype characterisation of two CF rat models
- McIntyre et al., Lobe-specific targeting of lentiviral vector gene transfer to the lungs of adult rats
Thoracic Society of Australia and New Zealand (Adelaide)
- Farrow et al., Does Conditioning the Airway Epithelium Improve Stem Cell Transplantation?
- Carpentieri et al., Comparative Efficiency of HA and VSV-G Pseudotyped Lentiviral Vectors for Cystic Fibrosis Airway Gene Therapy
- McIntyre et al., Generation of new cystic fibrosis rat models in Australia
American Society of Gene and Cell Therapy - Chicago, USA
- Farrow et al., Epithelial disruption enables human airway stem cell transplantation in mouse nasal airways
- Carpentieri et al., Airway gene-addition therapy for Cystic Fibrosis: The VSV-G pseudotype produces higher transduction levels than HA
2017
North American Cystic Fibrosis Conference - Indianapolis, USA
- Werdiger et al., Detection of regional lung disease in B-ENaC mice on a laboratory X-ray source
- McIntyre et al., Generation of new cystic fibrosis rat models in Australia developed using CRISPR/Cas9 genome editing
- Farrow et al., High efficiency lentiviral human airway basal cell transduction
- Carpentieri et al., Comparative efficiency of HA and VSV-G pseudotyped lentiviral vectors developed for treating cystic fibrosis lung disease
Australasian Cystic Fibrosis Conference - Melbourne
- McIntyre et al., New cystic fibrosis rat models in Australia developed using CRISPR/Cas9 genome editing
- McCarron et al., Assessment of bioreactor systems for up-scaling cystic fibrosis gene vector production
- Prichard et al., Perceptions of airway gene therapy for cystic fibrosis
- Carpentieri et al., Testing the efficiency of a ha pseudotyped lentiviral vector for treating cystic fibrosis lung disease
- Donnelley et al., Assessing the effectiveness of lung gene therapy using cystic fibrosis rats and x-ray pinpoint spirometry
Australian Gene and Cell Therapy Society - Sydney
- Donnelley et al., How do we know if cystic fibrosis lung gene therapy works? The importance of the right animal model, and the right measurement method
American Society of Gene and Cell Therapy - New Orleans, USA
- McCarron et al., Lentivirus Production in Stirred-Tank and Packed-Bed Basket Bioreactor Systems: A Comparison
Thoracic Society of Australia and New Zealand (Canberra)
- Farrow et al., Developing high efficiency gene transfer techniques using human air liquid interface cultures
2016
North American Cystic Fibrosis Conference - Orlando, USA
-
Donnelley et. al., Direct x-ray measurement of airway surface health in animal models: an update on the state-of-the-art
-
Cmielewski et. al., Rapid in vivo nasal transepithelial potential difference measurement in mice
American Society of Gene and Cell Therapy - Washington, USA
- McCarron et al., Development of a clinically-acceptable lentiviral vector for cystic fibrosis airway gene therapy
2015
Medical Applications of Synchrotron Radiation - Grenoble, France
- Donnelley et al., Assessing particle mucociliary transit excised sheep trachea at the Australian Synchrotron IMBL
North American Cystic Fibrosis Conference - Phoenix, USA
- Farrow et al., Cystic Fibrosis mouse models display age dependent airway basal stem cell hyperplasia
- Cmielewski et al., Delivery of human amniotic stem cells corrects CFTR function in CF mouse nasal airways In Vivo
Australian Cystic Fibrosis Conference - Sydney
- Donnelley et al., Assessing the effect of inhaled particle size on mucociliary transit activity in live mice
- Devereux et al., Airway conditioning enhances long-term lentiviral reporter gene expression in mouse lung
American Society of Gene and Cell Therapy - New Orleans, USA
- Devereux et al., Airway pre-treatment enhances mouse lung lentiviral reporter gene expression
- McIntyre et al., Comparing the efficacy of tat-dependent and tat-independent lentiviral vectors
2014
X-ray Microscopy - Melbourne
- Donnelley et. al., Non-invasive airway health measurement using synchrotron x-ray microscopy of high refractive index glass microbeads
North American Cystic Fibrosis Conference - Atlanta, USA
- Farrow et. al., Gene therapy for CF: is long term expression a consequence of transducing conducting airway endogenous respiratory stem cells?
International Society of Aerosol Medicine - Sydney
- Padmanabhan et. al., Improving the transduction efficiency of an aerosol-delivered lentiviral vector for cystic fibrosis lung gene therapy
Australian Society for Medical Research - Adelaide
- Padmanabhan et. al., Comparing the transduction efficiency of a liquid bolus and aerosol delivered lentiviral vector for cystic fibrosis lung gene therapy
American Society of Gene and Cell Therapy Conference - Washington, USA
- Cmielewski et. al., Lentiviral airway gene transfer in normal ferrets
2013
Australian Cystic Fibrosis Conference - Auckland, NZ
- Donnelley et. al., Advances in airway surface imaging for cystic fibrosis: Extended monitoring of individual particle mucociliary clearance
- Cmielewski et. al., Improved survival is by airway lentiviral CFTR gene transfer in a cystic fibrosis mouse model
Thoracic Society of Australia and New Zealand Conference - Darwin
- Cmielewski et. al., CF mouse survival is improved by lentiviral CFTR airway gene transfer
Stem Cells and Cell Therapies in Lung Biology Conference - Vermont, USA
- Bertoncello et. al., Evidence of an expanded and dysregulated airway epithelial stem cell compartment in cystic fibrosis mice
British Society of Gene and Cell Therapy Conference - London, UK
- Farrow, et. al., Endogenous stem/progenitor cell compartments of conducting airways differ in cystic fibrosis and normal mice
2012
North American Cystic Fibrosis Conference - Orlando, USA
- McQualter et. al., Endogenous lung epithelial stem/progenitor cell compartments differ in cystic fibrosis and normal mice
- Cmielewski et. al., Airway lentiviral CFTR gene transfer extends the lifetime of CF mice
American Society of Gene and Cell Therapy Conference - Philadelphia, USA
- Parsons et. al., High speed X-ray imaging reveals dosed-fluid dynamics and fate in mouse nasal and lung airways
Thoracic Society of Australia and New Zealand Conference - Canberra
- Donnelley et. al., High frame rate imaging of nasal fluid dosing dynamics
2011
North American Cystic Fibrosis Conference - Anaheim, USA
- Donnelley et. al., Dry deposition of pollutant and marker particles onto live mouse airway surfaces reveals heterogeneous mucociliary transit behaviour
- Cmielewski et. al., Bioluminescence gene expression with a lentiviral vector in airways of cystic fibrosis mice
Australian Cystic Fibrosis Conference - Melbourne
- Donnelley et. al., Mucociliary transit behaviour of pollutant and marker particles on live mouse airways
- Cmielewski et. al., Sustained reporter airway gene expression with a lentiviral vector in cystic fibrosis mice
Australian and New Zealand Society of Respiratory Scientists Meeting - Perth
- Cmielewski et. al., Differences in longevity of lentiviral luciferase expression in airways of normal and cystic fibrosis mice
- Donnelley et. al., Synchrotron X-ray imaging explains gene expression patters in mouse nasal airways
Thoracic Society of Australia and New Zealand Conference - Perth
- Donnelley et. al., Where does it go? Dynamically tracking the fate of fluid from lung instillations in mice
American Society of Gene and Cell Therapy Conference - Seattle, USA
- Parsons et. al., One year persistence from a single HIV-1 lentiviral vector delivery into marmoset lung: LacZ and vector gene presence
- Cmielewski et. al., Reporter gene expression following repeat administration of a HIV-1 lentiviral vector in mouse airways
- Donnelley et. al., Delivery dynamics and destination of gene vector instillations in live mouse airways
2010
North American Cystic Fibrosis Conference - Baltimore, USA
- Cmielewski et. al., Long term single dose gene correction of the bioelectrical CFTR gene defect in cystic fibrosis mice
European Cystic Fibrosis Conference - Valencia, Spain
- Donnelley et. al., High-resolution synchrotron X-ray imaging of live mouse airways: Overcoming challenges in physiological assessment
Thoracic Society of Australia and New Zealand Conference - Brisbane
- Cmielewski et. al., Repeated-measure analysis of lifetime lentiviral correction of the gene defect in cystic fibrosis mice
- Parsons et. al., Airway lentiviral gene transfer in marmosets
Australian Health and Medical Research Conference - Melbourne
- Cmielewski et. al., Long term gene expression with reporter and therapeutic lentiviral gene vectors in cystic fibrosis mice
Australian and New Zealand Society of Respiratory Scientists Meeting - Brisbane
- Cmielewski et. al., Differences in re-emergence of lung luciferase expression following nasal instillation of a lentiviral gene vector in normal and cystic fibrosis mice
American Society of Gene and Cell Therapy Conference - Washington, USA
- Parsons et. al., Airway lentiviral gene transfer in marmosets
2009
North American Cystic Fibrosis Conference - Minneapolis, USA
- Donnelley et. al., Individual particulate mucociliary transit analysis using synchrotron X-ray imaging
Australian Cystic Fibrosis Conference - Brisbane
- Donnelley et. al., Individual particulate mucociliary transit analysis using synchrotron X-ray imaging
- Parsons et. al., Progress and barriers toward lentiviral airway gene transfer development within three animal models
- Parsons et. al., Broadbanding CF: Patient, carer, clinical and research opportunities to improve CF life and care using internet enabled devices
American Society of Gene Therapy Conference - San Diego, USA
- Cmielewski et. al., Six months lentiviral correction of the CFTR gene defect in CF mice
Thoracic Society of Australia and New Zealand Conference - Darwin
- Donnelley et. al., Monitoring individual pollutant particle behaviour on intact live airways using synchrotron X-ray imaging
- Cmielewski et. al., Increased in-vivo sensitivity for transgene expression in murine nasal and lung airways using luciferase imaging
- Parsons, Respiratory research communications via the exclusive AARNet internet network
-
-
Recent News
New Australian Patent Application
Congratulations to David, Martin and Ali on their new Patent Application No. PCT/AU2021/051164 entitled “Methods and devices for delivering agents to the respiratory system”. This approach helps dramatically improve gene transfer effectiveness in the airways.MPS Foundation Grant: “Quantification of airway disease in MPS 1 mice via laboratory X-ray velocimetry”
Along with collaborators including Dr Emma Parkinson-Lawrence and Prof Sandra Orgeig, David and Martin were awarded ~$70k funding from the MPS Foundation in the USA to examine the lungs of mice with Hunter & Hurler syndrome, using X-ray Velocimetry.CF Foundation Grant: “Effective delivery of genetic therapeutics for cystic fibrosis airway disease”
Congratulations to Martin, Ali, David, Dr Chris Christou and clinical collaborator Dr Tom Goddard for being awarded a ~$400k from the USA CF Foundation to develop their novel physical airway surface perturbation method for enhancing gene transfer for CF.Australian Cystic Fibrosis Research Trust Innovation Grant: “Safety and effectiveness of triple -caftor combinations in cystic fibrosis during pregnancy”.
With new collaborators Dr Elena Schneider-Futschik and Dr Mark Habgood from the University of Melbourne the CFARG team have been awarded $80k to examine the effects of the CFTR modulator therapy Trikafta (recently approved for use on the PBS in April) on the developing foetus, and to assess whether the drug is effectively transferred via milk from the mother. This project is utilising the team's novel CF rat models.The Hospital Research Foundation Grant: “A simple rapid-production ventilator for COVID-19 treatment applications worldwide”
In 2020 at the beginning of the COVID-19 pandemic David and Martin were awarded $25k funding from THRF for ventilator development studies with industry collaborator 4DMedical. This resulting in the successful development, production and testing of a simple ventilator made from commonly available food-grade parts.November 2019: WCH Foundation Grant
Congratulations to Ali McCarron for being awarded a WCH Foundation grant worth $100k for her project “Airway gene correction in neonatal cystic fibrosis rats”. This project allows Ali to continue her exciting PhD program of work.November 2019: National Foundation For Medical Research and Innovation Grant.
Dr Martin Donnelley and A/Prof Parsons with their collaborators A/Prof Sarah Vreugde and Prof Peter Wormald at the TQEH have been awarded a NFMRI grant for their project “A novel treatment for Non-Tuberculous Mycobacteria lung infections in cystic fibrosis patients”!December 2019: NHMRC PFAS Targeted Call
David and Martin are part of team led by Prof Albert Juhasz at UniSA, who have been awarded $1.4M from the NHMRC for their project “Impact of exposure pathway and source on PFAS absorption and bioavailability”. This important project will help understand the biological impact of substances called PFAS that are commonly present in fire-fighting water.2019: National Imaging Facility funding
The ReXIL team were recently part of a successful National Imaging Facility (NIF) bid for XV animal scanning at SAHMRI. The application driven by A/Prof Parsons, Dr Martin Donnelley, and Dr Tim Kuchel was designed to provide equipment and core research capabilities for all Australian researchers to use, and was granted almost $1 M. They then received co-funding from the South Australian Government, the University of Adelaide, UniSA, The Hospital Research Foundation, the Hospital Services Charitable Gifts Board, and 4DMedical to fund the remainder. As a result $4.8M was committed for small and large animal scanners at the PIRL facility.April 2019: Australian Lung Health Initiative, MRFF Frontier Health and Medical Research Program Stage One Grant
The ReXIL team, as part of the Australian Lung Health Initiative (ALHI), have been awarded $960k from the Medical Research Future Fund for their project “4D Functional Diagnosis: A New Frontier In Lung Health For Children”. This funding will be used to collect the data required to build a business case for further large MRFF funding. This exciting project is a collaboration between multiple institutions including SAHMRI and Monash University, as well as industry partners 4DMedical and Micro-X. -
News
